- •Introduction
- •Figure 1 Post stroke disabilities (Genetech 2014)
- •87% Of strokes are ischemic, the rest are hemorrhagic.
- •Figure 4 Molecular process of neuronal ischaemia. (Colledge, Walker and Ralston., 2010, p. 1182)
- •Available treatments for stroke
- •Induced pluripotent stem cells
- •Mesenchymal stem cells
- •Figure 8 Variety of tissue types msCs have the potential to differentiate into, depending on signals produced by the local physiological environment. (Kishk et al., 2010)
- •MsCs for treatment of stroke
- •Migratory mechanisms of msCs
The underlying mechanisms of mesenchymal stem cell migration in ischemic stroke patients.
Abstract
Contents
|
Page 1 |
|
Page 1 |
|
Pages 2-3 |
|
Page 4 |
|
Page 5 |
|
|
|
|
|
|
|
|
10. Conclusion |
|
11. References |
|
Introduction
Every year, 15 million people worldwide suffer a stroke. Nearly six million die and another five million are left permanently disabled. (World Heart Federation, 2014) Stroke results from a rapid cessation in adequate amount of blood supply reaching sections of the brain. Presently, the therapies available are: thrombolysis, which is aimed at removing the obstruction by enzymatic digestion of the blood clot and thrombectomy, the surgical removal of the blood clot. However, first clinical trials have confirmed improved functional recovery in stroke patients after systemic delivery of bone marrow-derived mesenchymal stem cells (MSCs). (Doeppner et al, 2010)
This statement is supported by numerous animal studies, which used both human-MSCs and non-human-MSCs; such as those done by: (Mahmood et al., 2005), (Horita et al., 2006), (Lindcall et al., 2004) and (Zheng et al., 2009). Kim et al., (2013) also point out the benefits of MSC therapy in humans; however the exact mechanisms of migration of MSCs still remain unclear (Chamberlain et al., 2007). It is essential to understand the molecular mechanisms underlying MSC transfer within the bloodstream, penetration through the vascular wall and tissue invasion in order to develop a functional therapeutic method. The aim of this project is to identify the known migratory mechanisms of MSCs towards ischaemically damaged regions of the brain after systemic delivery and possible modifications improving MSC tropism and homing in the damaged regions.
Methods
What is stroke
The World Health Organisation has defined stroke as: “A neurological deficit of cerebrovascular cause that persists beyond 24 hours or is interrupted by death within 24 hours.”
Obstruction of the cerebral blood vessels and deprivation of oxygen, glucose and other essential nutrients to the supplied vascular territory, leads to cell death, through liquefactive necrosis. This is because brain tissue stops functioning if deprived of oxygen for more than 60 to 90 seconds. If circulation is not re-established within three hours, irreversible damage will be done to the affected area of the brain, causing it to lose function and can lead to visual impairment, inability to comprehend or formulate speech, move one or multiple limbs and other physical, cognitive and emotional problems resulting in acquired disability. (Figure 1)
Figure 1 Post stroke disabilities (Genetech 2014)
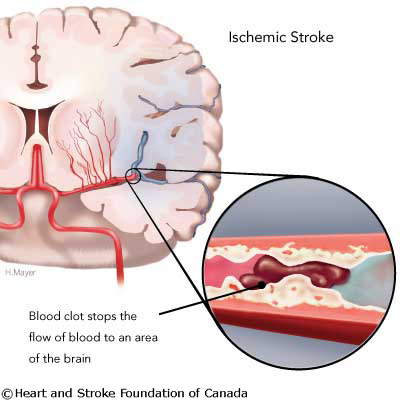
Two major types of stroke are: ∙ Ischaemic sroke (Figure 2)
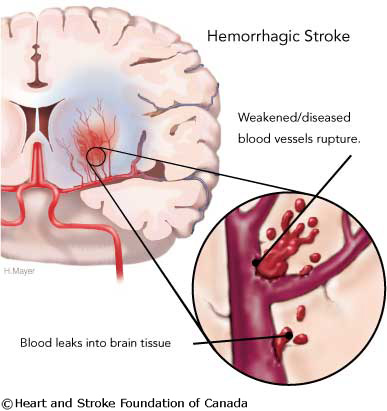
∙ Haemorrhagic stroke (Figure 3)
87% Of strokes are ischemic, the rest are hemorrhagic.
|
|
Figure 2 Ischaemic Stroke (Heart and stroke foundation of Canada, 2008)
|
Figure 3 Haemorrhagic Stroke (Heart and stroke foundation of Canada, 2008)
|
Explanations why ischaemic stroke can occur include:
|
|
Haemorrhagic strokes are intracerebral bleeds and can be caused by:
|
|
Stroke risk factors can be fixed or modifiable and include: (Colledge, Walker and Ralston., 2010, p. 1181)
|
|
Kazuhiko et al., (2011) also suggest that infection with serotype k Streptococcus mutants expressing collagen-binding protein is a potential risk factor for haemorrhagic stroke.
Molecular mechanisms of neuronal ischaemia and infarction are depicted in Figure 4 and occur in the following order:
Reduction of blood flow reduces supply of oxygen and glucose, hence ATP and H+ions.
Failure of energy-dependent membrane ionic pumps, leads to cytotoxic cerebral oedema and membrane depolarisation, which allows calcium entry and release of glutamate.
Calcium enters cells via glutamate-gated channels and activates destructive intracellular enzymes, destroying intracellular organelles and cell membrane with release of free radicals.
Free fatty acid release activates pro-coagulant pathways, which exacerbate local ischaemia.
Glial cells take up H+ions and thus cannot take up extracellular glutamate and suffer cell death.
The release of inflammatory mediators by microglia and astrocytes lead to necrosis of all cell types in the whole arterial territory.