- •Photosynthesis and the Chloroplast
- •8 Photosynthesis and the Chloroplast 173
- •The Light and Dark Reactions
- •Photosynthetic Units and Light-harvesting Assemblies
- •Localization of the z-Pathway Components
- •Organization of the Components of Photosystems I and
- •In the most frequently observed route, oxaloacetic acid is next reduced to malic acid in a reaction that uses nadp as an electron donor:
- •Integration of Chloroplast Activity in Plant Cells
- •The Blue-green Algae
Localization of the z-Pathway Components
Isolated chloroplasts are easily broken and separated into stroma and membrane fractions. The internal chloroplast membranes, originating from grana and stromal lamellae, contain the chlorophylls and carotenoids, the reaction centers and primary acceptors of photosystems I and II, and the enzymes and cofactors for electron transport, ATP synthesis, and the reduction of NADP. With the notable exception of CFi, which is relatively easy to dislodge from chloroplast membranes, the molecules of the Z-pathway and photophosphorylation are tightly bound to membrane structures as integral proteins and lipids.
Organization of the Components of Photosystems I and
II Complete disruption of inner chloroplast membranes releases both photosystems I and II. Using this technique, J. P. Thornber (reviewed in Thornber, 1975) and others have shown that the photosystems are released as high-molecular-weight
particles containing chlorophyll assemblies in association with reaction centers and some electron-transporting substances. Although the components vary with the particular methods used, an isolated photosystem II particle proves to contain a light-harvesting assembly combined with chlorophyll a and b in approximately equal quantities. Carotenoids are also present in the photosystem II complex, at levels approximating one-third to one-seventh of the amount of chlorophyll. The photosystem II particles also contain the reaction center for this system, chlorophyll P680, and cytochrome b3. Photosystem I particles contain both chlorophyll a and b in a ratio of about 20-30 chlorophyll a molecules to each chlorophyll b molecule. The reaction center chlorophyll of photosystem I, P700, can also be detected in this particle, with about one P700 molecule for every 45 chlorophyll a molecules. Depending on the isolation methods used, the photosystem I particles may also contain cytochrome f, cytochrome b6, plastocyanin, and < ferredoxin.
Complete disruption of inner chloroplast membranes also releases a large chlorophyll-protein particle that contains as much as 40-60% of the total chlorophyll content of chloro-plasts, including both chlorophylls a and b. The complex, now called the light-harvesting chlorophyll-protein or LHCP complex, has no reaction center and is apparently inactive in converting light energy to chemical energy. However, the LHCP complex is believed to act as a large antenna, absorbing and delivering quanta to the photosystems. Because the LHCP complex is most frequently found in close association with photosystem II, it is believed to pass the energy of absorbed quanta primarily to this photosystem.
Arrangement of Photosystems I and II and Electron Transport Carriers within Thylakoid Membranes The distribution of the components of the Z-pathway within thylakoid membranes has been analyzed by exposing isolated thylakoids to a variety of nonpenetrating agents, in experiments similar to the approach used to work out the topography of mitochon-drial membranes (see p. 159). The agents used (reviewed in DePierre and Ernster, 1977) include antibodies made against individual components, chemicals that label or modify parts of the system exposed at either membrane surface, artificial electron donors or acceptors, and digestion of exposed groups by specific lipase and protease enzymes. Since the agents used do not penetrate across membranes, reaction with intact thylakoids marks only the components of the Z-pathway facing the outer, stromal surface of the thylakoid membranes. Reaction with broken thylakoids marks the components facing the thylakoid compartment as well as the outer surface.
Both photosystems I and II are accessible to antibodies, enzymes, and chemical labels from both sides of thylakoid membranes and thus probably span the entire thylakoid bilayer. CFj, ferredoxin, and ferredoxin-NADP reductase (the flavoprotein linking ferredoxin and NADP) are all accessible to antibodies, enzymes, and markers in unbroken thylakoids,
indicating that these components are located in the bilayer half I1^
facing the stroma. Cytochrome f and plastocyanin react in bro- |1
ken thylakoids, indicating their distribution in the bilayer half I -
facing the thylakoid compartment. Since the plastoquinones J
are unavailable from either side, they are probably completely I
buried within the hydrophobic core of the membrane. These I
results establish that the components of the Z-pathway are I
distributed asymmetrically in thylakoid membranes, in the I probable arrangement shown in Fig. 8-17.
The Spatial Relationship of Z-Pathway Components to the I Mechanism Synthesizing ATP The asymmetric location of I the elements of the Z-pathway in thylakoid membranes and the fact that only some of the electron-transporting substances are dual hydrogen-electron carriers provide the basis for ATP synthesis according to the Mitchell hypothesis (see Hinkle and McCarty, 1978, and Mitchell, 1979). In chloroplasts, the first H+ ions added to the gradient are considered to be derived from the reaction splitting H2O, which is proposed to take place on the membrane surface facing the thylakoid compartment. Since chlorophyll P680 is a pure electron carrier, the 2H+ removed from H2O are released to enter the thylakoid com- I partment. The electrons removed from H2O, after excitation in the P680 reaction center, pass from P680 to the plastoquinones J of the primary acceptor pool. Plastoquinones are dual hydrogen-electron carriers, and the 2H+ required for the reduction of a plastoquinone molecule in the pool are derived from the solution on the stroma side of the thylakoid membrane. These H+ ions are expelled into the thylakoid compartment as the electrons pass from the plastoquinone to cytochrome f, a non-H+carrier located on the side of the membrane facing the thylakoid compartment. Mitchell has proposed that a plastoquinone-cytochrome cycle similar to the mitochondrial Q cycle (see pp. 155-156) may also operate at this point in chloroplasts to increase the number of H+ ions expelled across the membrane. From cytochrome f, the electrons pass to plastocyanin and the P700 reaction center of photosystem I. All of the carriers of this segment are non-H+ carriers. After excitation, the electrons pass through the short carrier chain to FAD, a dual H+-electron carrier facing the stroma. Reduction of FAD removes a second pair of H+ ions from the stroma, converting FAD to FADH2. The electrons and one of the 2H+ carried by the FAD are delivered to NADP at the end of the sequence, and the other H+ is released again into the stroma.
The total Z-pathway is thus considered at a minimum to remove 3H+ from the stroma and to expel 4H+ into the thylakoid compartment for each electron pair flowing from H2O to NADP. An additional 2H+ may be transferred from the stroma to the thylakoid compartment if a plastoquinone cycle operates in chloroplast electron transport as proposed by Mitchell. One H+ derived from the stroma remains linked to the NADP reduced in the last step. The H+ gradient created by the mechanism, low in the stroma and high inside the thylakoid compartment, provides the energy that drives ATP
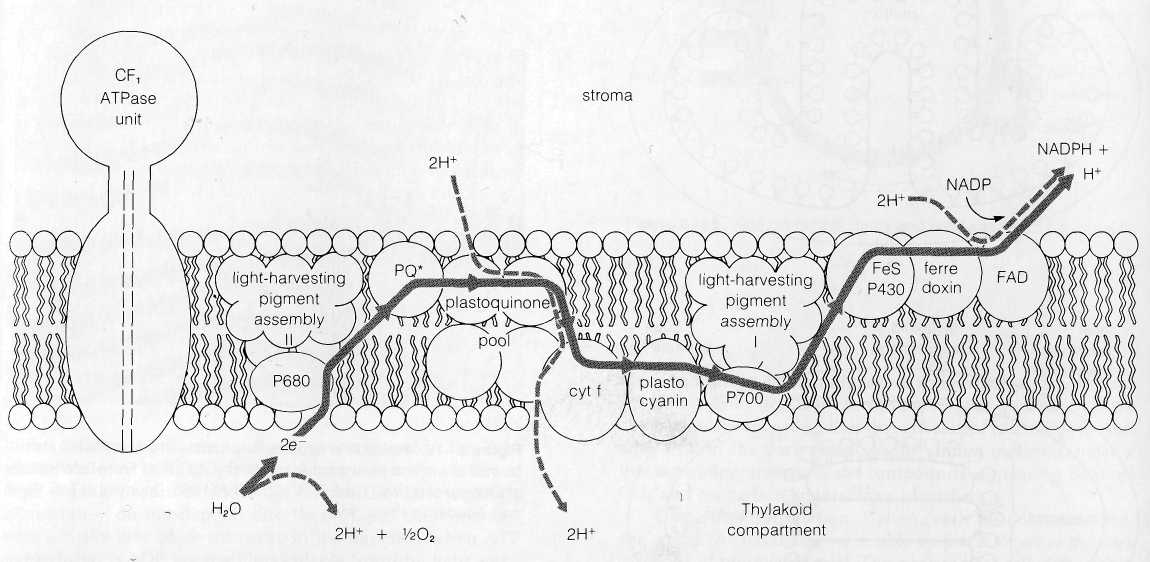
 Figure
8-17 The
asymmetric location of the components of the chloroplast electron
transport carriers in thylakoid
membranes. The hypothetical flow of electrons (solid lines) and H+
ions (dotted lines) to produce an H+
gradient
is shown in the diagram (see text).
Figure
8-17 The
asymmetric location of the components of the chloroplast electron
transport carriers in thylakoid
membranes. The hypothetical flow of electrons (solid lines) and H+
ions (dotted lines) to produce an H+
gradient
is shown in the diagram (see text).
 synthesis.
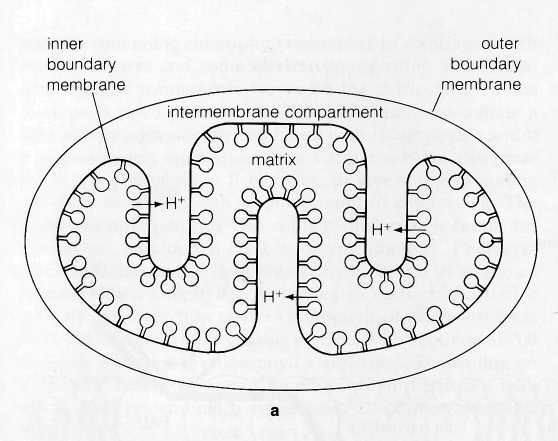
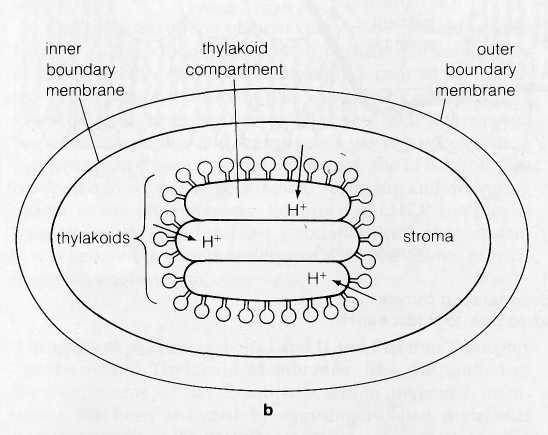
Extension of the ATPase lollipops into the stroma and
the direction of H+
ion expulsion in chloroplasts makes the
thylakoid compartment the functional equivalent of the
in-termembrane
compartment in mitochondria (Fig. 8-18).
synthesis.
Extension of the ATPase lollipops into the stroma and
the direction of H+
ion expulsion in chloroplasts makes the
thylakoid compartment the functional equivalent of the
in-termembrane
compartment in mitochondria (Fig. 8-18).
The total amount of ATP synthesized for each pair of electrons following the Z-pathway from H2O to NADP, as noted, remains in doubt. In the original experiments carried out by Arnon and his coworkers, apparently, one ATP molecule was synthesized for every electron pair. More recently, this ratio has gradually risen to fractional values between one and two ATP molecules per electron pair with improvements in the procedures used to isolate and maintain chloroplasts (see, for example, Hall et al., 1971). This has prompted the hypothesis that, in intact cells, each electron pair moving through the Z-pathway leads to the synthesis of two ATP molecules.
Thylakoid infrastructure and the Z-Pathway
Although sectioned thylakoid membranes' show evidence of extensive particulate substructure (Fig. 8-19), it has not been possible to relate the image seen in sections to either the photosystems or the electron transport carriers of the light reactions. Somewhat greater success has been achieved by application of the freeze-fracture technique (see the Appendix). This technique, which frequently splits membrane bilayers into inner and outer halves, exposes particles of two sizes inside the thylakoid membranes (Fig. 8-20). The small particles, about 11 nm in diameter, are distributed throughout both grana and stromal lamellae membranes. The large particles, 17
ran in diameter, are restricted to grana, probably to the membranes in regions where adjacent thylakoids join together.
Tracing the appearance of the 11- and 17-nm particles in developing chloroplasts has revealed the probable relationship of the particles to the two photosystems and the LHCP complex (see Henriques and Park, 1976, and Armond, Staehelin, and Arntzen, 1977). Thylakoids in the chloroplasts of immature leaves contain only the small particles, which are distributed throughout the membranes. Biochemical analysis shows that these thylakoids contain both photosystems but lack the LHCP complex. Upon maturing, the chloroplast inner membranes develop the LHCP complex, which becomes associated with photosystem II. At the same time, the large particles ap-
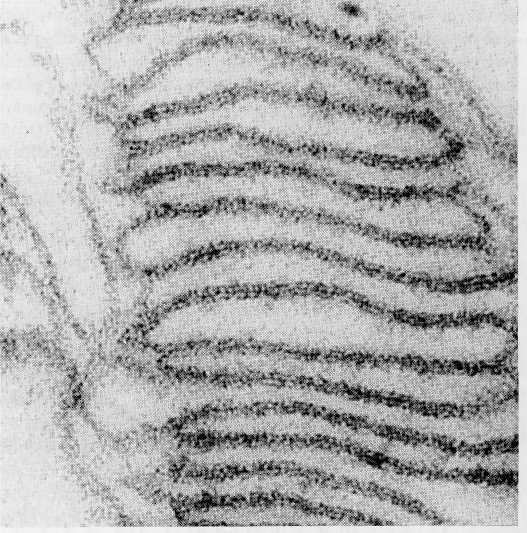
Figure 8-19 Participate substructure visible in the thylakoid membranes of a chloroplast from Aspidistra, x 197,000. FromBiochemistry of Chloroplasts, Vol. 1, ed. T. W. Goodwin, 1966. Courtesy of T. E. Weier and Academic Press, Inc.
i
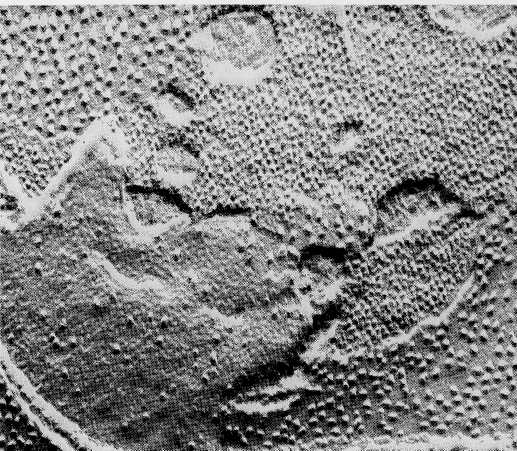
Figure 8-20 Freeze-fractured thylakoid membranes of spinach chlo-roplasts. Particles of two distinct sizes are exposed by fractures that split the bilayer halves, x 90,000. From Photophysiology, Vol. 3, ed. A. C. Giese, 1968. Courtesy of D. Branton and Academic Press, Inc.
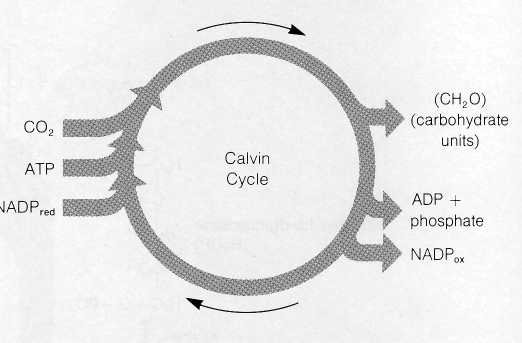
The light reactions, from the initial absorption of light to the production of ATP and reduced NADP (NADPH), provide energy (ATP) and reducing power (reduced NADP) for the dark reactions. ATP and reduced NADP accumulate after illumination of chloroplasts; unless they are used within a short time by the dark reactions, with the regeneration of oxidized NADP and ADP, the efficiency of photosynthesis falls.
Dark Reactions: CO2 Fixation in Photosynthesis
The Calvin Cycle
In the dark reactions, chemical energy produced in the light reactions is used to fix CO2 into carbohydrates and a variety of other organic products. Although termed the dark reactions because they do not depend directly on light, these interactions actually take place primarily in the daytime, when ATP and reduced NADP are readily available from the light reactions.
Little progress was made in unraveling the dark reactions until the 1940s, when radioactive tracers first became available to biochemists. One substance in particular, CO2 labeled with the radioactive isotope 14C, made possible the first real breakthroughs in this research.
M. Calvin, A. A. Benson, and their colleagues used this radioactive form of CO2 to trace out the biochemical pathways of the dark reactions. In their experiments, Calvin and his colleagues allowed photosynthesis to proceed in Chlorella in the presence of radioactive CO2. At various times after exposure to the labeled CO2, extracts of carbohydrates and other substances were made from the cells.
If the carbohydrate extracts were made within a few seconds after exposure, most of the radioactivity could be identified with the three-carbon sugar 3-phosphoglyceraldehyde (3-PGAL). The CO2 taken in by Chlorella was incorporated very rapidly into this three-carbon substance, evidently one of the earliest products of photosynthesis. If extracts were made after longer periods, radioactive label showed up in more complex substances, including a variety of six-carbon sugars, sucrose, and starch.
In other experiments, Calvin and his colleagues reduced the amount of CO2 so that photosynthesis could proceed only very slowly, even though adequate light was supplied. Under
Figure 8-21 The overall reactants and products of the Calvin cycle.
these conditions, a nonradioactive five-carbon sugar, ribulose-1,5-diphosphate (RuDP), accumulated in quantity in the chloroplasts, suggesting that this substance is the first to react with CO2 in the dark reactions. By similar methods, most of the remaining intermediate compounds appearing between CO2 and six-carbon sugars were identified.
Using this information, Calvin, with his colleagues Benson and J. A. Bassham, were able to piece together the dark reactions of photosynthesis. The cycle is now called the Calvin cycle, or the C3 cycle, because the first sugar product of the cycle is a triose or three-carbon sugar. Calvin was awarded the Nobel Prize in 1961 for his brilliant work in deducing the reactions of the cycle.
Reactions of the Calvin Cycle The reactions worked out by Calvin and his colleagues resemble the Krebs cycle in that the intermediate compounds of the sequence are continuously regenerated as the cycle turns. The cycle uses CO2, ATP, and reduced NADP as net reactants and releases ADP, oxidized NADP, and carbohydrates in the form of 3-PGAL as net products (Fig. 8-21).
In the first reaction of the cycle (Fig. 8-22), CO2 combines directly with the five-carbon, two-phosphate substance RuDP. The reaction, catalyzed by the enzyme ribulose-l,5-diphosphate carboxylase (RuDP carboxylase), produces two molecules of 3-phosphoglyceric acid, a three-carbon- substance. One of these contains the newly incorporated CO2 in the position marked by an asterisk in Fig. 8-22. The reaction requires no input of energy because the two three-carbon products exist at a much lower energy level than RuDP, which can be considered as a high-energy substance. The RuDP carboxylase enzyme catalyzing this reaction makes up as much as 25-50 % of the total protein of chloroplasts. This large quantity is attributed to the
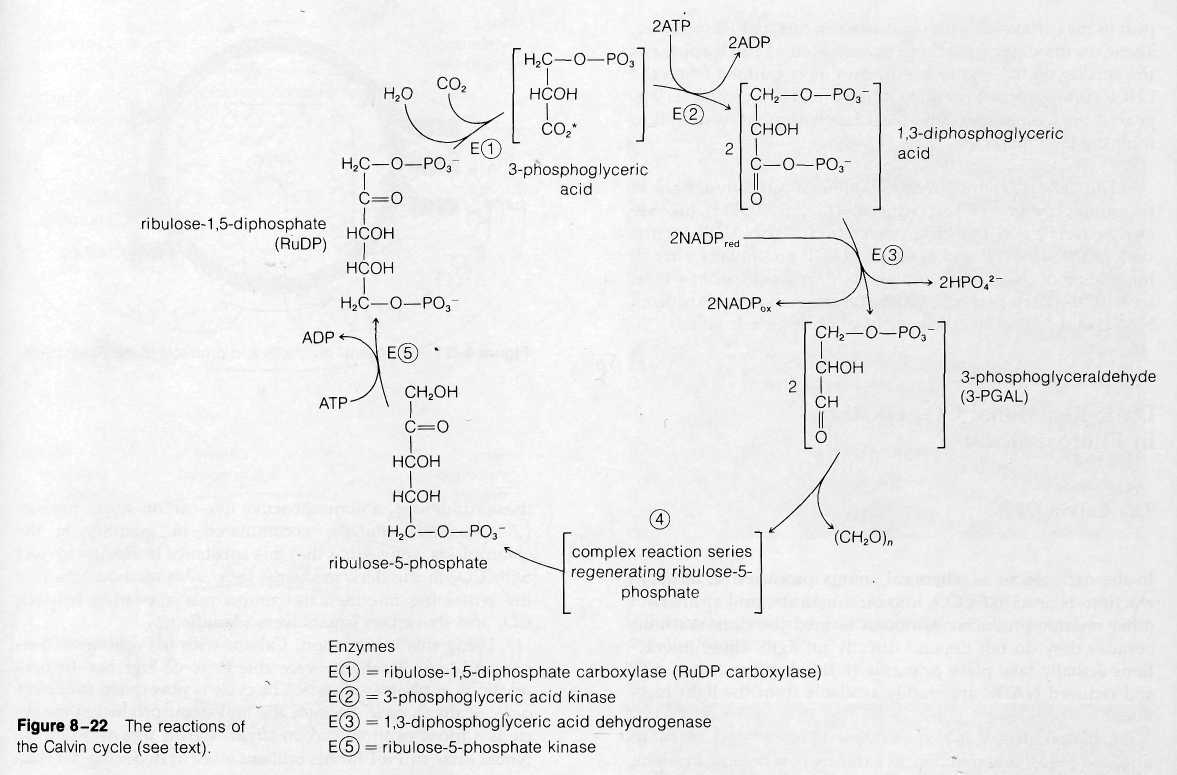
The next two reactions of the cycle, which proceed at the expense of ATP and reduced NADP, yield the net carbohydrate product of the cycle, 3-PGAL. In the first of these reactions, 3-phosphoglyceric acid is phosphorylated to form the more reactive substance 1,3-diphosphoglyceric acid. The phosphate added in the reaction is derived from ATP, which is converted to ADP in the process. This activated molecule accepts an H+ ion and two electrons from NADP in the next reaction, a reduction. One phosphate is removed from the substrate in the reaction, which yields 3-PGAL. For each molecule of CO2 attached to RuDP, two molecules of 3-PGAL are ultimately produced. The total reaction sequence to this point uses two ATP molecules and two reduced NADP molecules:
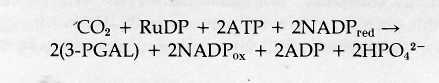
Some of the 3-PGAL produced at this step is required to regenerate the RuDP used in the first step in the cycle; some is released as a surplus to the cycle to enter the major biochemical pathways leading to glucose, sucrose, starch, and other complex products of photosynthesis.
Regeneration of RuDP occurs through a complex of reactions (not shown in Fig. 8-22) that yields as an initial product the five-carbon, one-phosphate sugar ribulose-5-phosphate. This product is then phosphorylated to RuDP at the expense of one additional ATP. This reaction regenerates the RuDP used in the first step in the cycle, and the entire series is ready to turn again.
Three turns of the Calvin cycle are required to yield one molecule of 3-PGAL as a surplus that can be used for synthesis of more complex carbohydrates. In three turns of the cycle through reaction 3 in Fig. 8-22, six molecules of 3-PGAL are formed (for a total of 18 carbons). Five of these molecules (containing 15 carbons) enter the complex series that regenerates the three RuDP molecules used in the three turns. The remaining molecule of 3-PGAL is surplus and can enter synthetic
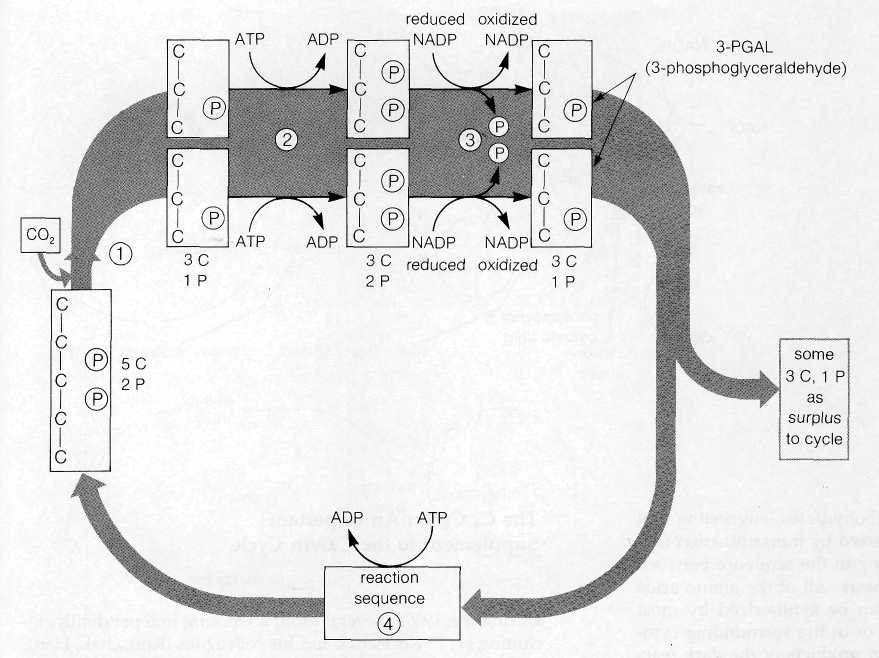
pathways forming more complex substances. A single turn of the cycle can thus be regarded as taking up one molecule of CO2 and yielding one unit of carbohydrate, (CH2O). Six turns of the cycle are required to make enough carbohydrate units to yield one molecule of a hexose such as glucose.
For each turn of the cycle, a total of two ATP and two reduced NADP molecules are used in steps 2 and 3 in Fig. 8-22. One additional ATP molecule enters the cycle in the reaction regenerating RuDP, for a total of three ATP molecules for each turn. As net reactants and products, one complete turn of the cycle therefore includes:
![]()
Fig. 8-23 summarizes the reactions of the cyclein simplified form.
Formation of Complex Sugars and Starch from 3-PGAL The
primary product of the Calvin cycle, 3-PGAL, is the starting point for synthesis of a variety of complex carbohydrates and
polysaccharides. Glucose and other hexoses are formed through a series of reactions that essentially reverse part of glycolysis (see Fig.'7-4). In glycolysis, two reactions between glucose and 3-PGAL are essentially irreversible. These reactions are run in the reverse direction, synthesizing glucose from 3-PGAL, by the activity of enzymes that replace the gly^lytic enzymes at these points.
Note from Fig. 7-4 that no extra ATP is required to synthesize glucose from two molecules of 3-PGAL. Sufficient energy is contained in 3-PGAL to drive the reaction sequence in reverse when the triose sugars are present in high concentration. And, although inorganic phosphate is released as a part of the sequence from 3-PGAL to glucose, none is incorporated into ATP. Thus, there is no net change in the amount of ATP in the synthesis of glucose from 3-PGAL.
Free glucose is actually formed in only very limited quantities in the chloroplasts of most plants. Instead, glucose-1-phosphate and other hexose-1-phosphates are used as the starting points for synthesis of sucrose, starch, cellulose, and a wide variety of additional organic molecules. In addition to carbohydrates, amino acids and proteins also become labeled rapidly in illuminated chloroplasts supplied with radioactive CO2. In fact, label frequently shows up in amino acids before it
appears in the more complex carbohydrates, suggesting that amino acids are probably synthesized by transamination (see also p. 160) of intermediates falling in the sequence between CO2 reduction and glucose synthesis. All of the amino acids required for protein synthesis can be synthesized by most plants, either inside chloroplasts or in the surrounding cytoplasm, by pathways starting from products of the dark reactions. Protein synthesis, occurring on ribosomes suspended in the chloroplast stroma, can also be detected inside chloroplasts, along with polymerization of nucleotides into DNA and RNA (see Supplements 12-2 and 13-1).
After a few minutes of photosynthesis in labeled CO2, lipids within chloroplasts also become labeled. In some cases, synthesis of labeled lipids, including fatty acids, fats, and galactolipids, may represent as much as 30% of the total labeled material inside chloroplasts after 1-2 min of photosy#-thesis. This lipid synthesis includes the various photosynthetic pigments: Chloroplasts are able to carry out all of the reactions required to make chlorophylls and carotenoids from simple precursors originating from the dark reactions of the Calvin cycle.
The balance among carbohydrates, fats, and amino acids synthesized in chloroplasts varies widely among different species of plants. In some, nearly all of the CO2 absorbed is incorporated into carbohydrates such as sucrose, formed from glucose and fructose. In others, such as the alga Chlorella, the synthesis of fats and amino acids greatly exceeds sucrose synthesis, which may account for 5% or less of the CO2 absorbed. In any event, the chloroplasts of most species contain the enzymes and intermediates required for synthesis of a wide variety of substances in addition to carbohydrates and indeed have synthetic capacity practically equivalent to entire cells.
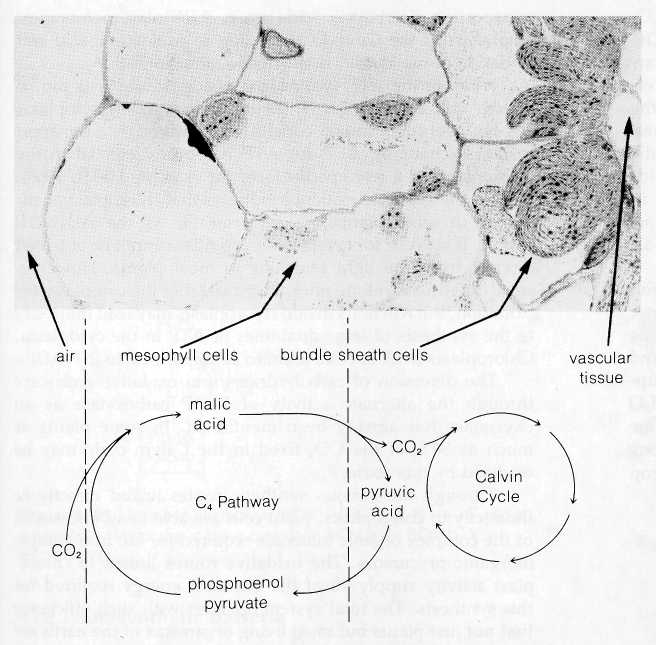
The C4 Cycle: An Important Supplement to the Calvin Cycle
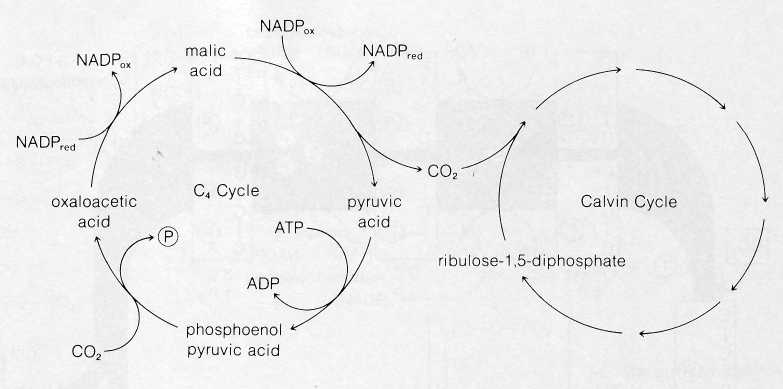
During the 1960s, several groups working independently, including H. P. Kortschak and his colleagues (Kortschak, Hartt, and Burr, 1965) and M. D. Hatch and C. R. Slack (1966), discovered that certain plants are able to carry out a supplemental series of reactions that apparently improves the efficiency of CO2 utilization by the Calvin cycle. This pathway, now called the C4 cycle, was discovered when the Kortschak group and Hatch and Slack looked for the earliest-labeled intermediates produced in corn, sugar cane, and other tropical grasses after the plants were exposed to labeled CO2. Surprisingly, the earliest label appeared in a pool of malic and other four-carbon acids instead of 3-PGAL as in the Calvin cycle. Intermediates of the Calvin cycle were also found to be labeled a few seconds after the appearance of label in the four-carbon acids.
The
product of this carboxylation follows a subsequent pathway
that varies among different plants using the C4
cycle.
![]()