
- •I.P. Volchok, s.B. Belikov, V.V. Gazha
- •I.P. Volchok, s.B. Belikov, V.V. Gazha, 2008
- •Contents
- •Preface
- •1 Structural materials
- •1.1. Classification and General Properties of Structural Materials
- •Fig. 1.2. The major groups of engineering materials
- •1.2. Mechanical Properties
- •Fig. 1.8. Principle of Brinell hardness test:
- •1.3. Atomic-Crystal Structure of Metals
- •Fig. 1.20. Edge dislocation in a crystal lattice
- •1.4. Solidification and Metal Structure
- •Fig. 1.25. Cooling curves for a pure metal
- •1.5. Phase Diagrams and Structure of Alloys. System of Iron-Carbon Alloys
- •1.6. Heat-Treatment of Steel
- •1.7. Chemical Heat-Treatment (Casehardening) of Steel
- •1.8. Classification and Identification of Iron-Carbon Alloys
- •2 Metallurgy
- •2.1. Materials Used in Metallurgy
- •2.2. Blast-Furnace Process
- •2.3. Steel production
- •2.4. Production of Non-Ferrous Metals
- •2.5. Powder metallurgy
- •3 Foundry practice
- •3.1. Theoretical Fundamentals of Foundry
- •3.2. Manufacture of Castings in Sand Moulds
- •3.3 Shell-Moulding Process
- •3.4. Metal Mould Casting
- •3.5. Centrifugal Casting (Spinning)
- •3.6. Pressure-Die Casting
- •3.7. Investment Casting
- •3.8. Modern Processes of Metal Production for Castings
- •4 Metal forming
- •4.1. Physical and Mechanical Fundamentals of Metal Forming
- •4.2 Recovery and Recrystallization
- •4.3. Technological Plasticity
- •4.4. Heating of Metals
- •4.5. Rolling
- •4.6. Extrusion of Metals
- •4.7. Drawing
- •4.8. Hammering
- •4.9. Die Forging
- •4.10 Stamping
- •5 Welding
- •5.1. The Physical Fundamentals of Welding
- •5.2. Arc Welding
- •5.3. Gas Welding
- •5.4. Resistance Welding
- •5.5. Diffusion Welding
- •6 Metal cutting operations
- •6.1. Principles of Cutting and Shaping the Metals
- •6.2 Geometry of a Cutting Tool
- •6.3. Cutting Speed and Chip Formation
- •6.4. Cutting Materials
- •6.5. Machine Tools Classification
- •6.6. Lathe Works
- •6.7. Drilling
- •6.8. Planing, Shaping and Slotting
- •6.9. Milling
- •6.10. Gear - Cutting Methods
- •6.11. Grinding
- •6.12. Finishing and Microfinishing Processes in Machining of Metals
- •6.13 Electrophysical and Electrochemical Machining
- •Dictionary
- •Bibliography
4.2 Recovery and Recrystallization
The process of recovery occurs, when a strain-hardened metal is heated to comparatively low temperatures, usually below (0.2 or 0.3) T melting point (Tmp). It comprises the relief of microstresses and partial elimination of distortion of the crystal lattice as a result of reduction in density of structural defects.
The ordinary set of mechanical properties does not usually exhibit any changes in the recovery process.
A further rise in temperature increases the mobility of the atoms, and when a definite temperature is reached, new equiaxed grains are formed (Fig. 4.4). The formation of new equiaxed grains in place of the oriented fibrous structure is called recrystallization treatment or primary recrystallization. As a result of recrystallization the effects of strain hardening are practically completely eliminated; and the properties approach their initial values. As may be seen in Figure 4.4, the tensile strength and especially the yield point drop sharply upon recrystallization, and the ductility ( and ) increases. This reduction in strength and hardness can be explained by the elimination of lattice distortion and the drastic reduction in dislocation density (from 1010…1012 to 106…108 cm-2). The lowest temperature tbr, at which recrystallization begins and proceeds and at which metal is softened is called the temperature threshold of recrystallization.
The temperature tbr is about 0.4 Tmp for commercially pure metals. For pure metals this temperature is reduced to (0.1 to 0.3) Tmp and for solid solution alloys it is raised to (0.5 to 0.6) Tmp. As the first appoxitation this rule enables the temperature at which primary recrystallization begins to be determined. It is about -33°C for lead, about 270°C for copper and about 450°C for low-carbon steel. These temperatures separate cold and hot metal working.
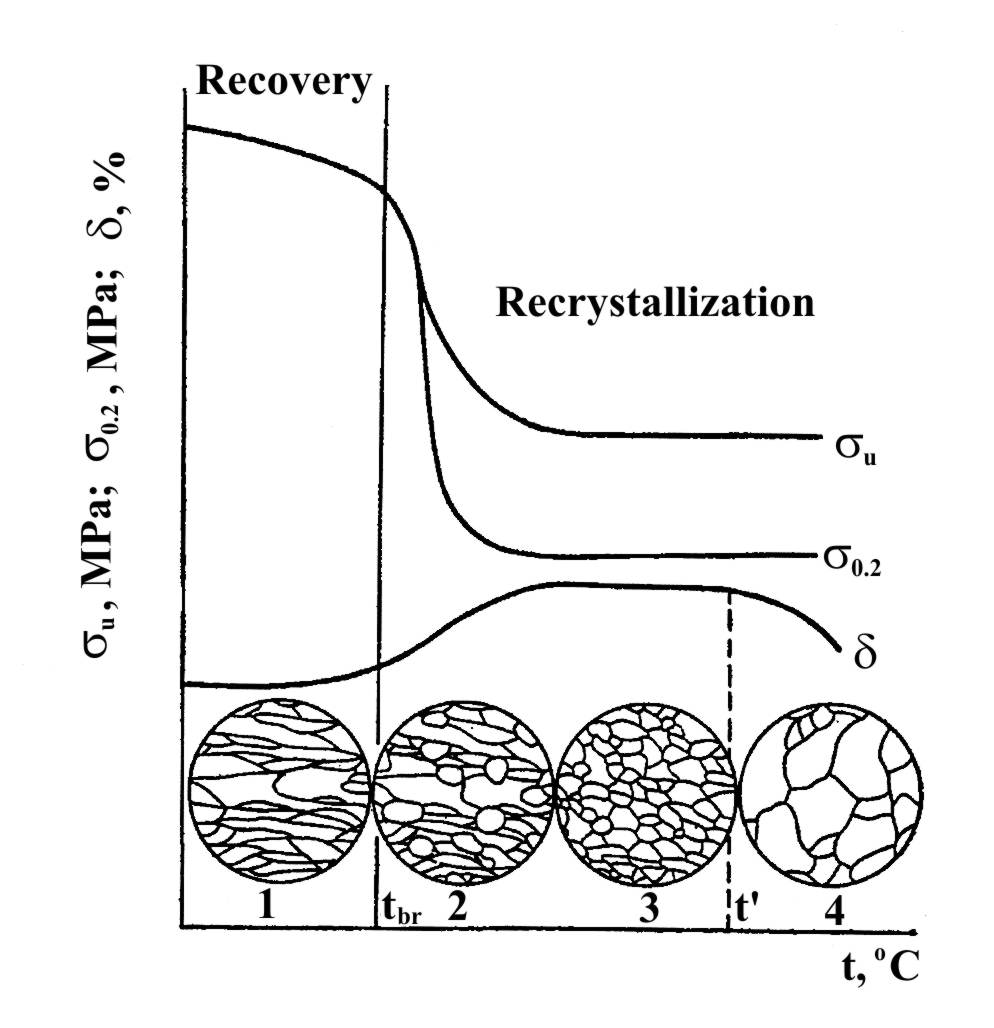
Fig. 4.4. Effect of heating on the mechanical properties and structure of strain-hardened steel
To eliminate the effect of strain hardening metal is heated to higher temperatures than tbr to ensure a high rate of recrystallization and completion of the process. Such heat-treatment is called recrystallization annealing (it is carried out at temperatures from 650 to 750°C for steel).
Collective recrvstallization. When the process of primary recrystallization has been completed, subsequent heating leads to growth of recrystallized grains. This process is called collective recrystallization. In collective recrystallization period the mechanical properties are reduced because of large sizes of grains.
Overheating and Burning. Prolonged heating of steel at temperature above t (Fig.4.4) leads to the formation of exceptionally large actual grains, both at these temperatures and after cooling to 20 °C. This effect is called overheating. Overheated steel has a coarse crystalline fracture and low mechanical properties. Overheating can be corrected by repeated heat treatment.
Heating steel to a higher temperature than that causing overheating, especially in an oxidizing atmosphere, leads to burning. This is accompanied by the formation of iron oxides along the grain boundaries. Burnt steel has a stony brittle fracture. Burning is an irremediable defect of steel.
