
- •I.P. Volchok, s.B. Belikov, V.V. Gazha
- •I.P. Volchok, s.B. Belikov, V.V. Gazha, 2008
- •Contents
- •Preface
- •1 Structural materials
- •1.1. Classification and General Properties of Structural Materials
- •Fig. 1.2. The major groups of engineering materials
- •1.2. Mechanical Properties
- •Fig. 1.8. Principle of Brinell hardness test:
- •1.3. Atomic-Crystal Structure of Metals
- •Fig. 1.20. Edge dislocation in a crystal lattice
- •1.4. Solidification and Metal Structure
- •Fig. 1.25. Cooling curves for a pure metal
- •1.5. Phase Diagrams and Structure of Alloys. System of Iron-Carbon Alloys
- •1.6. Heat-Treatment of Steel
- •1.7. Chemical Heat-Treatment (Casehardening) of Steel
- •1.8. Classification and Identification of Iron-Carbon Alloys
- •2 Metallurgy
- •2.1. Materials Used in Metallurgy
- •2.2. Blast-Furnace Process
- •2.3. Steel production
- •2.4. Production of Non-Ferrous Metals
- •2.5. Powder metallurgy
- •3 Foundry practice
- •3.1. Theoretical Fundamentals of Foundry
- •3.2. Manufacture of Castings in Sand Moulds
- •3.3 Shell-Moulding Process
- •3.4. Metal Mould Casting
- •3.5. Centrifugal Casting (Spinning)
- •3.6. Pressure-Die Casting
- •3.7. Investment Casting
- •3.8. Modern Processes of Metal Production for Castings
- •4 Metal forming
- •4.1. Physical and Mechanical Fundamentals of Metal Forming
- •4.2 Recovery and Recrystallization
- •4.3. Technological Plasticity
- •4.4. Heating of Metals
- •4.5. Rolling
- •4.6. Extrusion of Metals
- •4.7. Drawing
- •4.8. Hammering
- •4.9. Die Forging
- •4.10 Stamping
- •5 Welding
- •5.1. The Physical Fundamentals of Welding
- •5.2. Arc Welding
- •5.3. Gas Welding
- •5.4. Resistance Welding
- •5.5. Diffusion Welding
- •6 Metal cutting operations
- •6.1. Principles of Cutting and Shaping the Metals
- •6.2 Geometry of a Cutting Tool
- •6.3. Cutting Speed and Chip Formation
- •6.4. Cutting Materials
- •6.5. Machine Tools Classification
- •6.6. Lathe Works
- •6.7. Drilling
- •6.8. Planing, Shaping and Slotting
- •6.9. Milling
- •6.10. Gear - Cutting Methods
- •6.11. Grinding
- •6.12. Finishing and Microfinishing Processes in Machining of Metals
- •6.13 Electrophysical and Electrochemical Machining
- •Dictionary
- •Bibliography
2.2. Blast-Furnace Process
Blast-furnace process is used in ferrous metallurgy for cast iron production. The main product of ferrous metallurgy is steel, but two-stage process of steel production is now predominantly used in the metallurgy: Fe-orecast ironsteel.
Cast iron (iron) is a general term applied to iron-carbon alloys, containing more than 2.14 %C.
So iron is obtained in blast furnace by reducing from ores by carbon. The following raw materials, named charge, are commonly used in the blast furnace process: iron ore, fuel, flux.
Four chief types of iron ore are used:
- hematite Fe2O3;
- limonite 2Fe2O3·3H2O;
- magnetite Fe3O4;
- siderite FeCO3;
After mining the iron ore is crushed to powder, dressed from impurities and sintered in pieces. Such sintered ore is called agglomerate. During agglomeration main part of sulphur is removed from the ore and limestone CaCO3 is added to the ore. Hence, we receive and use in blast furnace so-called fluxed ore.
The main fuel in blast furnace is coke, which is produced of coking coal by preheating it at temperature ~ 1000°C without air during 14…16 hours. Coke has the chemical composition: 80…88 %C, 8…12 % ash, 2…5 % moisture, 0.5…1.8%S, 0.02…0.2 %P. Part of coke may be replaced by natural gas (CH4), or black mineral oil, or powder coal, or blast furnace gas.
Limestone CaCO3 is used as a flux in the blast furnace.
The modern blast furnace constitutes the largest and most complicated type of metallurgical plant. Such a plant is capable to produce more than ten thousand tons of iron a day and night (24 hours). It works continuously from 7 to 10 years.
The blast furnace is like a vertical pipe, lining by refractory inside, in which fluxed ore and coke, named a charge, are charged from the top and preheated air (1100°C) is blown into the furnace below. Iron and slag are tapped from the furnace periodically through a tap hole and a slag hole.
The blast furnace derived its name from the fact that air to support combustion must be blown into it under pressure, because of the resistance offered by the column of material within the shaft to passage of the combustion gases. A typical blast furnace is shown in Fig. 2.1.
Chemical reactions between carbon, oxygen, iron and its oxides occur within the blast-furnace by combustion of coke and temperature equal from 1500 to 2000°C.
Nearby tuyeres carbon of coke combines with oxygen of air with evolution of heat:
![]() (2.1)
(2.1)
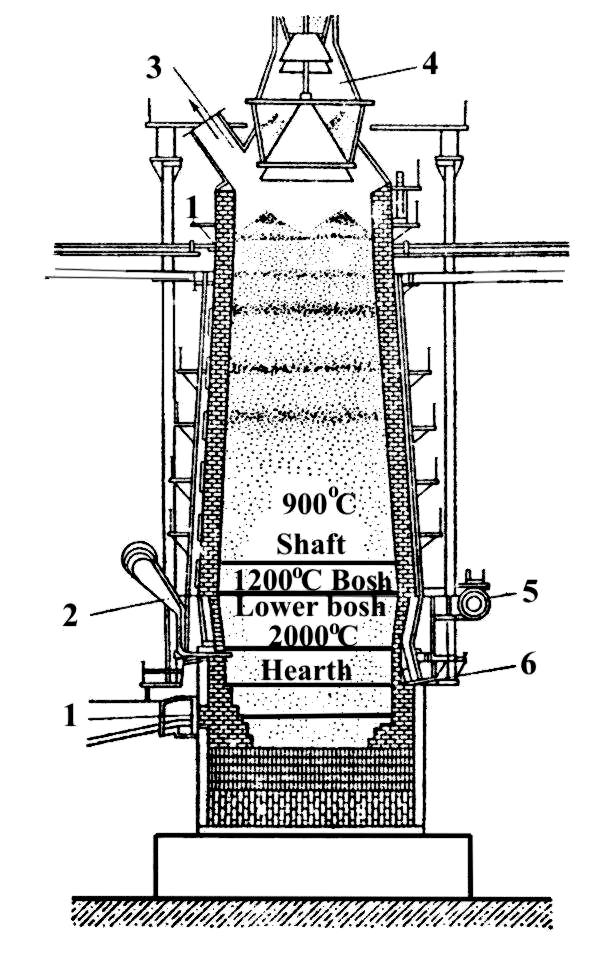
Fig. 2.1. Blast furnace: 1 – iron taphole; 2 – tuyeres; 3 – exhaust pipes; 4 – top;
5 – air blast pipe; 6 – slag hole
Reduction of iron is performed in the first turn by CO in succession from higher to lower oxydes and to pure iron (Fe2O3Fe3O4FeOFe):
![]() (2.2)
(2.2)
![]() (2.3)
(2.3)
![]() (2.4)
(2.4)
Reduction by CO is called indirect one, reduction by C and H2 is called direct one:
![]() (2.5)
(2.5)
![]() (2.6)
(2.6)
At temperature above 1000°C the carburizing of iron takes place:
![]() (2.7)
(2.7)
![]() (2.8)
(2.8)
Hence, because of the carburizing we have cast iron with approximately 4 % of carbon instead of pure iron.
The reduction of Mn, Si, P also takes place, and S from coke dissolves in molten cast iron. As a result, cast iron has the following chemical composition: 4.0…4.4 %C, 0.6…3.0 %Si, 0.3…1.0 %Mn, 0.15…0.30 %P, 0.03…0.07 %S.
The blast-furnace produces:
- conversion iron, or steelmaking pig iron, or pig iron used for steel-making practice (contains ~l %Si);
- foundry iron, poured in pigs and used for remelting in foundry shops (contains ~3 %Si);
- ferromanganese - alloys used for deoxidation and for alloying of steel. FeMn has average chemical composition: 7 % C, 70 % Mn, the rest-Fe;
- ferrosilicon - alloy used for deoxidation and alloying of steel: 2%C, 13%Si, the rest-Fe;
- slag (CaO, MgO, A12O3, SiO2, FeO, MnO, etc.) used in building industry;
- blast-furnace gas (14…18 % CO2, 22…28 % CO, 2…6 %H2, 50…55% N2) has low calorific value (3350…4000 kJ/m3).
