
Chemical properties of halogens
Fluorine
Fluorine gas is especionally chemically active; Fluorine molecule is the strongest chemical oxidant that has:
Low energy of F2 2F dissociation (159 kJ/mol). This energy is low due to the strong repulsion of nonbonding electrons in the molecule:
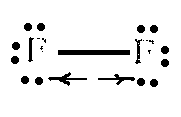
For instance, O2 dissociation energy is 493.2 kJ/mol and Cl2 dissociation requires 242.4 kJ.
High affinity to the electron F + e- F‑ (332.7 kJ/mol). This value is lower than in case of chlorine but dissociation energy compensates it.
These two factors (as well as small size of fluorine atom) determine a greater strength of covalent bonds of fluorine with other elements. For example, H—F bond energy is equal to 564.3 kJ/mol against 459.8 kJ/mol for H—O bond and 430,5 kJ/mol for H—Cl.
Coal, paper, and wood burn in fluorine atmosphere. The stable substances: glass (in the form of wool) as well as water self-ignite in fluorine either:
SiO2 + 2 F2 SiF4 + O2 (O3 also forms); H = -657 kJ/mol
2H2O + 2F2 4HF + O2 (O3 is co-product); H = - 598 kJ/mol
Therefore, dissolution of gaseous F2 in water is impossible. In addition, F2 substitutes hydrogen in hydrogen compounds of other elements.
Interaction of fluorine with many elementary substances proceeds vigorously. It interacts with H2 even at -220 oC (this temperature characterises transition to solid hydrogen). Fluorine reacts with sulfur and phosphorus at -190 oC (liquid air temperature).
S + 3F2 SF6 H = - 1207 kJ/mol
2P + 5F2 2PF5
Most metals (uncluding Au, Pt) burn in the atmosphere of fluorine on cold or at heating. At the same time, fluorine has no interaction with metallic Fe, Cu, Ni, Al, and Zn in ambient conditions. This unexpected behaviour can be explained by very tight protective coatings of fluorides formed on that metals surface.
When heating, fluorine oxidizes heavy noble gases (Kr, Xe, Rn), for example:
Xe + 3F2 XeF6
Elementary fluorine gas is inert towards n2 and o2, as well as He, Ar, Ne.
Fluorine substitutes other halogens in their compounds
NaI + F2 NaF + I2
All binary compounds of fluorine belong to one class of fluorides, in which fluorine is found in the oxidation state -1. Binary fluorides are divided obviously enough into ionic (NaF, MgF2) and covalent (SiF4, ClF3). There are also a few compounds with the intermediate (ionic-covalent) character of bond (ZnF2, MnF2, CoF2, NiF2). Ionic fluorides are basic, and the covalent fluorides have acidic nature:
SiF4 (acidic fluoride) + 2NaF (basic) = Na2SiF6
For comparison purposes, a reaction of interaction of basic and acid oxides is shown below:
Li2O + CO2 (acid oxide) = Li2CO3
Basic fluorides form alkaline medium due to hydrolysis:
NaF + H2O
![]() HF + NaOH
HF + NaOH
and acidic fluorides, in turn, provide acid environment:
SiF4 + 3H2O H2SiO3 + 4HF
Compounds of fluorine
Hydrogen fluoride. Fluorine gas interacts with H2 even at low temperatures explosively:
H2 + F2 = 2HF; H = - 535 kJ g/mol
This direct synthesis has no practical significance for HF preparation. However, it can be the source of rocket fuel. The flame temperature at burning of F2 + H2 mixture exceeds 4000 oC.
Obtaining (in industry). This is the fluorite reaction with concentrated H2SO4
CaF2 + H2SO4 = CaSO4 + 2HF (at 120-300 oC)

HF molecule is strongly polar [ = 1.91 D = 0.64.10-29 C.m]. This value exceeds the value of electric dipole moment of water, SO2 and NH3. A molecule of HF has strong tendency to association as a result of formation of the closely-coupled chain hydrogen bonds.
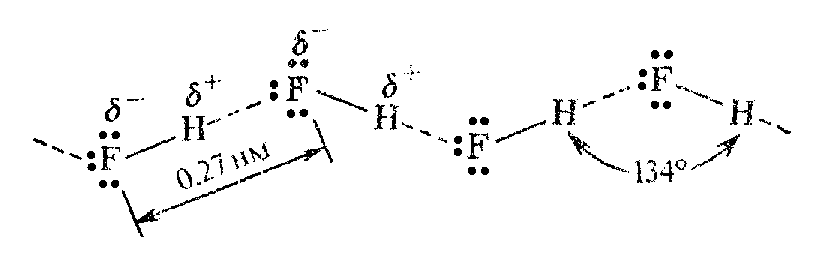 It
should be noted that formation of hydrogen bonds is
determined by: 1) extraordinarily small size of the positively
charged atom of hydrogen; 2) ability of hydrogen to penetrate deeply
into the electronic shell of neighbouring atom (that doesn’t form
covalent bond with it). Hereupon, electrostatic attraction together
with donor-acceptor interaction is the reason of
hydrogen bond formation.
It
should be noted that formation of hydrogen bonds is
determined by: 1) extraordinarily small size of the positively
charged atom of hydrogen; 2) ability of hydrogen to penetrate deeply
into the electronic shell of neighbouring atom (that doesn’t form
covalent bond with it). Hereupon, electrostatic attraction together
with donor-acceptor interaction is the reason of
hydrogen bond formation.
Hydrogen bond energy in HF is a little bit stronger (33 kJ g/mol) than in H2O molecules. That is why HF consists of mixture of polymers (HF)2, (HF)3, (HF)4, (HF)5, (HF)6 even in the gaseous state. B.p. measurements show that hydrogen fluoride molecules have average composition (HF)4.
Inorganic compounds mostly are well soluble in liquid HF. As a rule, such solutions are good conductors of electric current. Liquid HF is a strong ionising solvent. The dissolved substance accepts protons of HF molecules, increases HF2- ions concentration so it behaves as a base, for example:
KNO3 + 2HF K+ + HNO3 + HF2-
Chemical activity of HF depends substantially on the absence or presence even traces of water. Dry HF has no reaction with many metals and with metal oxides. On the other hand, when a reaction with an oxide will start even to negligibly small degree, it proceeds with autoacceleration farther as a result of the reaction:
MO + 2HF = MF2 + H2O
since the content of water grows.
HF dissolves in water without limits and a lot of heat (58,5 kJ/mol) liberates. The formation of azeotropic mixture (that boils without separation of components) takes place that contains 38.3% of hydrogen fluoride and boils at 112 oC.
Ionization of HF molecules occurs at dissolution in water with H3O+ and F- ions formation. The latter interact with HF molecules, as a result, hydrogen fluoride-ions are formed:
HF + H2O H3O+ + F- K = 7.2.10-4 CH3COOH
HF + F - HF2- K = 5.1 - due to hydrogen bonds
or total equation,
2HF + H2O H3O+ + H F2.-
Water solution of HF (hydrofluoric acid) is acid of intermediate strength. Its interesting feature is ability to interact with glass and quartz:
SiO2 + HF = SiF4 + 2H2O
Therefore it is forbidden to store it in glass bottles. For this purposes bottles should be coated with polyethylene, lead or rubber. HF is used for chemical etching of glass, making on it various pictures.
Mixture of concentrated HF and HNO3 (1:3) is stronger than “aqua regia” and able to dissolve Si, W, Ta, Mo, and Nb with formation of complex acid of these metals:
3Ta + 2IHF + 5HNO3 = 3H2[TaF7] + 5NO + 10H2O
FLUORIDES of OXYGEN. At 100-1000 К fluorine does not react directly with O2. Unlike other halogens fluorine has no oxygencontaining acids (single exception being HFO synthesized recently).
Compounds of fluorine with oxygen are not stable and exist only at low temperatures. Fluorine is the most electronegative in these compounds therefore oxygen carries a positive charge (+2). For this reason they are named «fluorides of oxygen». Fluorides of oxygen are endothermic compounds of fluorine:
Compound |
O F2 |
O2F2 |
O3F2 |
Hof, 298, kJ g/mol |
+ 16.7 |
+ 21.2 |
+ 26.1 |
Among them OF2 exists only room temperature– light yellow gas with a characteristic smell of fluorine, it dissolves very sparingly in water, is unstable, less active than fluorine, however it is a very strong oxidant. The covalent molecule OF2 has triangular structure: d (F—O) = 0.142, FOF = 103o. OF2 is formed when gaseous fluorine passes through cold dilute solution of alkali:
2![]() +
2
+
2
![]() =
=
![]() + 2
+ 2![]() + H2O
(1-2% solution of NaOH)
+ H2O
(1-2% solution of NaOH)
1e.2 4e
In this case we put coefficients before an oxidant and product of oxidation (it can be put before reductant and the product of reduction)
Fluoride of oxygen OF2 can be considered as an anhydride of hypofluorous acid HFO.
It reacts with non-metals, metals, inorganic and organic compounds. With water and alkalis it enters into the reaction of selfreduction-selfoxidation:
![]() + 2
+ 2![]() = 2HF +
= 2HF +
![]()
+ 2![]() = 2NaF +
+ H2O
= 2NaF +
+ H2O
Other fluorides of oxygen OnF2 (n = 2-6) are obtained due to the effect of high-voltage electric discharge on the mixture of oxygen and fluorine under the diminished pressure and low temperatures. All of them decompose completely at low temperatures.
O2F2 is an orange toxic gas (dF-O = 0.1575 nm, dO-O = 0.1217 nm; OOF 109,5о). m.p. = -154оC, b.p. = -57 oC, the half-decay period O2F2 at -50оС is 3 hours.
All fluorides of oxygen are very strong oxidants and fluorinating agents. So, O2F2 reacts with Xe, with many metals (among them Ag, Au, Pt). It forms salts of dioxygenyls with fluorides, for example:
PtF4 + O2F2 = O2PtF6 (O2+ -dioxygenyl)
The length of bond O—O (0.1123 nm) in it is less than in O2 (0.1207 nm). All dioxygenyls contain unpaired electron, all of them are therefore paramagnetics. The best studied among them is O2PtF6 forming orange-red crystals, m.p. = 219оC (with decomposition).
