
- •Electronic Configurations & Oxidation States
- •Physical properties
- •Physical Properties. Summary Some Alkaline-Earth Metals Subgroup Trends
- •History Of Discovery
- •Preparation
- •In industry:
- •Alkaline-earth metals chemical properties (1)
- •Alkaline-earth metals chemical properties (2)
- •Alkaline-earth metals chemical properties (3) Compounds hydrides
- •Tests for alkali and alkaline-earth metals subgroups elements
- •Hardness of water and its removal
Tests for alkali and alkaline-earth metals subgroups elements
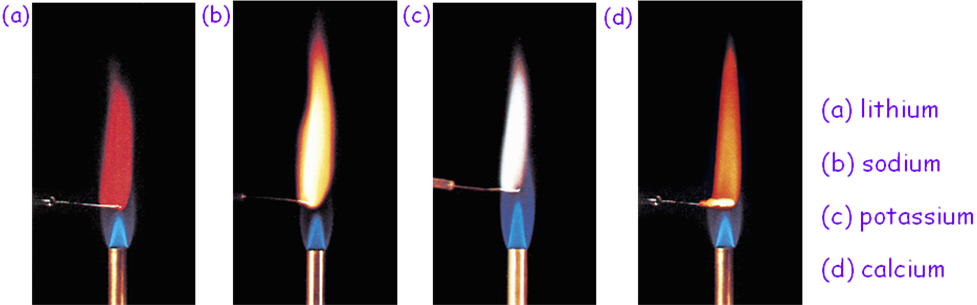
Group I element |
Flame colour |
|
Group II element |
Flame colour |
Li Na K Rb Cs |
Deep red Golden Yellow Lilac Bluish red Blue |
|
Ca Sr
Ba |
Brick red Blood red or Crimson Green |
Hardness of water and its removal
Natural water contains a small concentration of dissolved salts of Mg and Са, which predetermine its hardness. The formation of scum (scale) at boiling is an enormous deficiency of such water, that results in severe destructive losses (heat conductivity and coefficient of thermal expansion of scum substantially differ from the same parameters for metals). Therefore such water when utilized requires to be softened.
Temporal and permanent hardness of water are recognized.
Temporal hardness is determined by the presence of dissolved Са, Mg and Fe hydrocarbonates, and permanent - of their chlorides and sulfates. The latter at boiling remains in solution, while hydrocarbonates are decomposed:
Са(Mg,Fe)(НСО3)2 = Са(Mg,Fe)СО3 + СО2 + Н2О
Hardness is measured by the number of milligram-equivalents of each of metals in 1 l of water (1 mg-equiv. Са = 20 mg = 2·10-5 kg; 1 mg-equiv. Fe = 28 mg = 2.8·10-5 kg) or number of molar equivalent the masses (g-equiv.) per 1 m3 of water.
The sum of temporal hardness and permanent hardness makes up general water hardness. Water can be: soft, if its value is < 4, average hard (4-8), hard (8-12) and very hard (> 12).
Knowing the concentration of ions [Ca2+], [Mg2+], [Fe2+] in g-ion, general hardness can be found according to the formula:
Т = 2·1000· ([Ca2+] + [Mg2+] + [Fe2+])
It is multiplied by 1000 for translation of grams into milligrams, and by 2 - to pass from g-ions to the g-equivalent. For the bivalent metals 1 g-ion corresponds to two gram-equivalents.
Methods of water softening. Temporal hardness is removed by boiling:
М(НСО3)2 = МСО3 + СО2 + Н2О (М = Ca, Mg, Fe)
If water contains a lot of hydrogen carbonates, it is softened by addition of an equivalent amount of lime water:
М(НСО3)2 + Са(ОН)2 = МСО3 + СаСО3 + 2Н2О
For the removal of permanent water hardness lime is usually added, which precipitates Mg(OH)2, and then soda, which precipitates СаСО3.
For water softening, sometimes Na3PO4 is applied, instead of soda, which precipitates Mg2+ and Са2+ ions in insoluble phosphates. It is yet better to soften water by sodium polymethaphosphates known under the name of calgo. With the Mg, Ca, Fe ions the latter forms stable soluble complexes. Scum is not formed here, while already formed - dissolves.
Effective softening of water is possible to achieve by passing it through cationites
2Na—R + M2+ M(R)2 + 2Na+
After water treatment the cationite is processed with NaCl solution, shifting the resulted equilibrium to the left making it again suitable to application.
Resins which sorb aniones are named anionites. They contain amino groups NR2 in their composition, which contact with the molecules of Н2О with the formation of mobile ОН- ions. These ions can be substituted with anions, which are present in natural hard water:
R(NR3OH)x + yCl- R(NR3OH)x-y•(NH3Cl)y + yOH-
