
- •Electronic Configurations & Oxidation States
- •Physical properties
- •Physical Properties. Summary Some Alkaline-Earth Metals Subgroup Trends
- •History Of Discovery
- •Preparation
- •In industry:
- •Alkaline-earth metals chemical properties (1)
- •Alkaline-earth metals chemical properties (2)
- •Alkaline-earth metals chemical properties (3) Compounds hydrides
- •Tests for alkali and alkaline-earth metals subgroups elements
- •Hardness of water and its removal
Alkaline-earth metals chemical properties (3) Compounds hydrides
G roup
II elements Mg, Ca, Sr, and Ba react with H2
(g) to form hydrides
of
the formula MH2:
roup
II elements Mg, Ca, Sr, and Ba react with H2
(g) to form hydrides
of
the formula MH2:
Ca(s) + H2(g) = CaH2 (s)
CaH2 + 2H2O = Ca(OH) 2 + 2H2 (g)
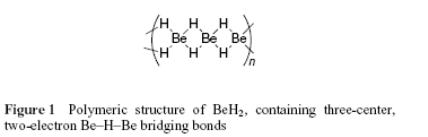
Heavier metals group II dihydrides (CaH2, SrH2, and BaH2) are ionic, containing hydride ion (H−). Beryllium and magnesium dihydrides are covalent and polymeric.
(BeH2)n demonstrates an interesting structural feature. In the gas phase, several species may be probably present, comprising polymeric chains and rings.
The solid state is polymeric, and the single-crystal X-ray diffraction structure indicates that it consists of the chains of beryllium atoms containing hydrogen bridges between them (see the figure below).
OXIDES. BeO and mgO. Simplest way to prepare oxides type Met=O is carbonates roasting:
Ве(Mg)СО3 Ве(Mg)О + СО2
Thus ВеСО3 begins to decompose already at higher than 100C, and MgCO3 – nearly at 500C. The use of natural magnesite MgСО3 for obtaining СО2 and MgO is based on this property. СО2 obtained so is very pure and is used in food industry.
BeO and MgO are white refractory solids with ionic structure type NaCl (m.p. BeO 2470C, m.p. MgO 2850С).
BeO is amphoteric, air-fluffy, MgO is mainly basic.
The heavier these oxides will be dissolved in acids, the stronger was preliminary roasting. It is explained by enlargement of its crystals. High temperature roasted BeO is not practically dissolved in aqueous solutions of acids and alkalis and with great difficulty it is dissolved only in melts of acidic salts (for example in KHSO4) or alkalis:
BeO + 2KOH K2BeO2 + H2O
BeO + 2KHSO4 BeSO4 + K2SO4 + H2O
After roasting MgO also becomes sufficiently chemically inert. On the contrary, formed at low temperatures, it slowly reacts even with water and with carbon dioxide:
MgO + H2O = Mg(OH)2
MgO + CO2 = MgCO3
MgO is an important raw material for obtaining various refractants (materials which stand heating to highest temperatures), and also for artificial building materials.
—
Ca, Sr, Ba. The reaction of Ca, Sr, Ba with oxygen leads to the formation with of white refractory (extremely thermally stable) oxides МО. In industry they are obtained by thermal decomposition of carbonates.
The pecularity of these metals is that they have the greatest enthalpies of formation, as compared to other elements in the calculation per 1 mole-atom of oxygen. Hereupon alkaline-earth metals easily reduce other elements from its oxides.
CaО, SrО, BaО react with water with a considerable elimination of heat. When СаО (called lime or quicklime, unslaked lime, quicklime) is mixed, or slaked with water Са(OH)2 (traditionally called slaked lime, hydrated lime, slack lime, or pickling lime) is obtained:
СаО + Н2О = Са(ОН)2 Н298 = -67 kJ/mol
Technologically this process is referred to as slaking (quick)lime. Са(OH)2, that is formed, is the cheapest and the most frequently used in industry strong base.
If at slaking quicklime 2 weight parts of СаО are replaced with 1 weight part of aqueous solution of NaOH, the so-called “natronic lime” (a mixture of Са(OH)2 with NaOH) is formed. This white powder is used in laboratories for СО2 absorption .
PEROXIDES, МeО2, are white hardly soluble solid compounds. The most used one is ВаО2, which is obtained at ВаО heating in the stream of air at 500С:
2ВаО + О2 = 2ВаО2, Н298 = -71.6 kJ/mol
It is determined by the fact that the Gibbs energy of ВаО2 formation (-587,9 kJ/mol) is greater than that for ВаО (-528.4 kJ/mol).
Peroxides of other metals are obtained according to the reaction:
М(ОН)2 + Н2О2 = МО2 + 2Н2О
They are salts of the very weak acid Н2О2; therefore they are decomposed (hydrolyzed) with water:
BaO2 + H2O Ba(OH)2 + Н2О2
BaO2 + H2O Ba2+ +2OH- + Н2О2
The reaction equilibrium is completely displaced to the right in acidic medium. Therefore it was earlier used for obtaining Н2О2.
ВаО2 demonstrates strong oxidizing properties which are determined by the decomposition of ВаО2 at higher than 700С:
2ВаО2 = 2ВаО + О2
HYDROXIDES. Be and mg. Be(OH)2 and Mg(OH)2 are white amorphous solids, they are very slightly soluble (solubility products are equal to 2·10-22 and 6·10-10 at 25C, respectively).
Be(OH)2 is a very weak electrolyte, Mg(OH)2 is also a weak electrolyte, but water suspension of Mg(OH)2 has weak alkaline properties.
Be(OH)2 is a typically amphoteric compound. Its soluble part dissociates poorly at basic or acid type, therefore Be(OH)2 easily dissolves in acids and alkalis:
Ве(ОН)2 + 2H+ = [Ве(Н2O)4]2+
Ве(ОН)2 + 2ОН- = [Be(OH)4]2-
tetrahydroxoberillate
In obedience to modern data, the reason for Be(OH)2 amphotericity (as well as for hydroxides of other multicharge cations of large polarizing action: Al3+,Cr3 and others like that) is the process of [Be(OH)4]2- complex anion formation but no ”neutralization of hypothetical acid Н2ВеО2 by an alkali ”, like it is shown below:
Ве(ОН)2 + 2NaOH = Na2BeO2 + 2H2O.
A complex compound Na2[Be(OH)4] dissolves well, as unlike Ве(ОН)2 it is not a polymer.
Mg(OH)2 dissolves in acids. Its interesting feature is dissolution in solutions of ammonium salts, for example:
Mg(OH)2 + 2NH4Cl = MgCl2 + NH4OH
It is determined by the fact, that Mg(OH)2 dissociates stronger than NH4OH. For Be(OH)2 such interaction is not characteristic.
An important feature of Be(OH)2, which allows to separate Be(OH)2 and Al(OH)3, is its good solubility in (NH4)2CO3 solution as a result of complex compound formation:
Be(OH)2 + 2(NH4)2CO3 = (NH4)2[Be(CO3)2] + 2NH4OH
At heating Be(OH)2 loses water already at 230, while Mg(OH)2 decomposes at weak-white glow incandescence.
Salts of Be and Mg generally are well dissolved in water. The hydrated ions of Ве(Н2О)42+ and Mg(Н2О)62+ are colorless.
Ion Ве2+ gives sweet tasted solution, and Mg2+ has a bitter taste.
All compounds of Be are very poisonous.
In water solutions Mg salts, and especially salts of Be, sufficiently strongly hydrolyze. Due to the small size of Ве2+ this ion strongly polarizes the molecules of water, which surround Ве2+, converting water into a strong acid (pK ~ 2):
Ве2+(Н2О) [Be(OH)]+ + H+.
The products of hydrolysis here can be found in solution without forming sediment. Research has shown that generally hydrolysis proceeds according to the equation:
3[Ве(Н2O)4]2+ [Be(H2O)2OH]33+ + 3Н+ + 3Н2О
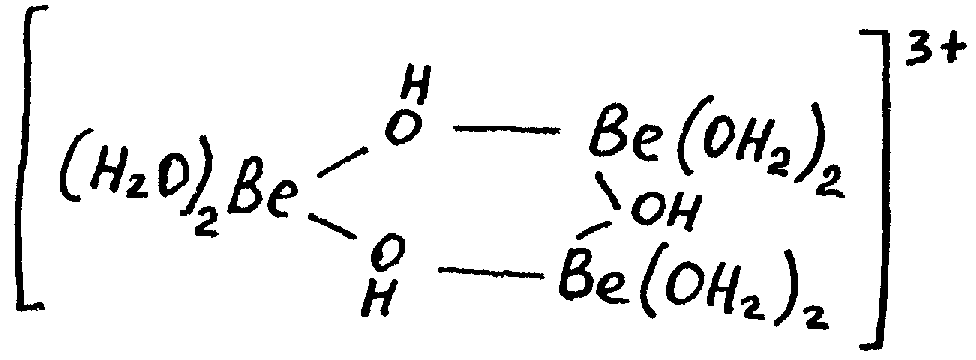
The equilibrium of hydrolysis reaction can be shifted to the right by addition of alkali. But precipitation of insoluble basic salts or Be(OH)2 begins only after that, when the quantity of ions OH- in solution starts to exceed 1 mol per 1 mol of Ве2+ ions. For the same reasons Be(OH)2 is well dissolved in solutions of its salts.
Ca, Sr, Ba, Ra. All Ca, Sr, Ba oxides and hydroxides are typical basic compounds that show chemical interactions of such compounds (with acid oxides, acids, amphoteric oxides, hydroxides, and salts). Their basicity is increased towards barium:
CaO + CO2 = CaCO3
CaO + 2HCl = CaCl2 + H2O
CaO + Al2O3 = Ca(AlO2)2
Ca(OH)2 + 2HNO3 = Ca(NO3)2 + 2H2O
Ca(OH)2 + 2Al(OH)3 = Ca[Al(OH4)]2
Ca(OH)2 + CuSO4 = Cu(OH)2 + CaSO4
Solubility in water going from Са(OH)2 to Ва(OH)2 grows considerably. It has a different character for Са(OH)2, Sr(OH)2, and Ва(OH)2. Solubility of Са(OH)2 at heating diminishes and this is a relatively rare example of solid compound solubility diminishment at the increase of temperature. On the contrary, Sr(OH)2 and Вa(OH)2 are dissolved better at heating and it explains the ease of theirs recrystallization from aqueous solutions.
A suspension of fine calcium hydroxide particles in water is called milk of lime. This suspension/solution is called lime water, it is a medium strength base that reacts violently with acids and attacks many metals in the presence of water. Lime water strongly absorbs СО2 from air precipitating СаСО3.
Са(OH)2 application
The main application is in construction industry as “lime solution”, or “mortar” (a workable paste used to bind construction blocks together and fill the gaps between them. The blocks may be stone, brick, cinder blocks, etc.).
“Lime solution” is prepared with 1 part of lime Са(OH)2 and 3-4 parts of sand adding water with the formation of doughy mass. It gradually hardens due to the formation of crystal hydrates of calcium Са(ОН)2•Н2О, and formation of crystalline СаСО3 with carbonic acid of air and crystalline silicates as a result of superficial interaction between Са(OH)2 and SiO2.
Са(OH)2 is used in sugar refining, where it helps to precipitate the additional amounts of sugar as calcium saccharate from molasses, that remains after separation of crystallic sugar.
It is also applied for production of chlorinated lime:
Ca(OH)2 + Cl2 = Ca(OCl)Cl
(mixed salt of HCl and hypochlorous acids). Chlorinated lime is a white powder that has strong oxidizing properties, and is used mainly for disinfections.
SALTS. Be and mg. Because of high capability of Ве2+ and Mg2+ cations to hydrolysis it is difficult to convert them into the corresponding carbonates. At the action of alkali metals carbonates on soluble salts of Be and Mg only basic carbonates are precipitated:
2MgSO4 + 2Na2CO3 + H2O = (MgOH)2CO3 + CO2 + Na2SO4
Basic carbonates are transformed into carbonates only when treated with concentrated solution КНСО3 at heating:
Mg(OH)2CO3 + 2KHCO3 2MgCO3 + K2CO3 + H2O
The water free Mg(ClO4)2 (“anhydron”) is a very effective means for drainage of gases or dessicant (efficiency is similar to Р2О5). It is prepared by dehydration of chrystalhydrate Mg(ClO4)2·6H2O at heating in vacuum at 240C. At higher than 380C it decomposes thermally.
Be and Mg halides are formed at interaction of elements. Crystalhydrates are precipitated from solutions: BeCl2·4H2O and MgCl2·6H2O. At heating those compounds split up not only water but also HHal, transforming into insoluble basic salts:
MgCl2.6H2O Mg(OН)Cl + HCl + 5H2O
BeCl2 is a hygroscopic compound which hydrolyzes easily. It is a typical inorganic polymer with bridge bonds:
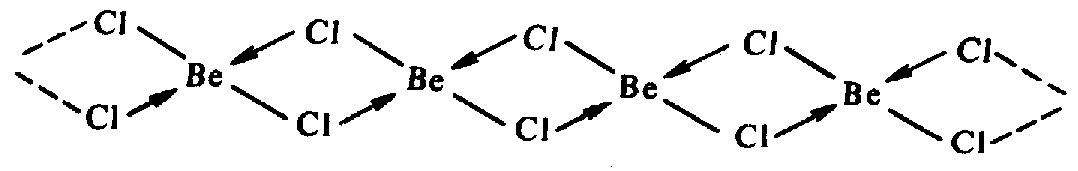
In addition, covalency of Be in this solid compound is equal to 4. Two bonds are formed according to the exchange mechanism by two unpaired electrons of the atom Be (in excited state) and two ones from the atom Cl. Two other bonds are formed according to the donor-acceptor mechanism from two vacant 2p-orbitals of Be (acceptor) and two lone electronic pairs of the atom Cl.
Molecules of BeCl2 in the gaseous state have a linear structure due to sp-hybridization of the Be atom.
CEMENT. It is by far the best comparative with lime solution astringent (binding) material. It hardens quickly, gives a considerably harder stone and does not produce house dampness which is long observed after application of lime solution. There are a few varieties of cement, some of them can harden under very special conditions (even under water).
Cement is produced by roasting the mixture of limestone and clay in huge rotary furnaces at t ~ 1400С. At exit from a furnace the alloyed mass (clinker) is obtained, which after cooling is finely ground. In such conditions an ordinary silicate (portland-) cement is produced. It is a greenish-grey powder which is the mixture of silicates and alum silicates of calcium, mainly: Ca3SiO5, Ca2SiO4, Ca3(AlO3)2.
The composition of cement is expressed by the ratio between the content (% by mass) of basic oxides (СаО) to acidic ones (SiO2 + Al2O3 + Fe2O3). The ratio СаО/[SiO2+ Al2O3 + Fe2O3] is the hydraulic module. It characterizes technical parameters of cement and for a silicate cement is equal to ~ 2. The composition of silicate cement therefore is, %: СаО (63), MgO (1.5), SiO2 (22), Al2O3 (6), Fe2O3. Other components are Na2O, K2O, SO3.
Being mixed with water cement gives mass, that gradually (for a few hours) hardens (“jumps”). Addition of sand to it, hogging and other fillers improve qualities of the artificial stone (concrete), which forms.
Hardening of silicate cement is the process of formation of crystal hydrates, whose crystals form hard stoning mass, as well as partially the surface reaction of Са(ОН)2 with SiO2:
Ca3SiO5 + 5H2O = Ca2SiO4·4H2O + Ca(OH)2
Ca2SiO4 + 2H2O = Ca2SiO4·2H2O
Ca3(AlO3)2 + 6H2O = Ca3(AlO3)2·6H2O
The use of aluma cement which contains 40 % СаО and Al2O3,10 % by mass Fe2O3 and SiO2 is also made. The major compound in it is Сa(AlO2)2. It hardens as a result of the process:
2Ca(AlO2)2 + 10H2O = Ca2Al2O5·7H2O +2Al(OH)3
SALTS OF OXYGENCONTAINING ACIDS. There is no hydracids for which salts of alkali metals are not known. These salts are crystalline, and stable compounds at STP.
Carbonates. These salts of alkaline-earth metals are practically insoluble in water, and they are minerals widely represented in nature. Most frequently used are all the varieties of the limestone СаСО3. From it quicklime (CaO) is produced which than is mainly converted into slaked lime Са(OH)2 . The latter is obtained in enormous quantities (tens of milliones of tons worldwide)
СаСО3 chemical decomposition is accompanied by considerable absorption of heat:
СаСО3 СаО + СО2; Но = 178 kJ/mol
The reaction has reversible character.
СаСО3, as well as the majority of other carbonates, is easily decomposed by acids which substitute Н2СО3. Its solubility in water rises when ammonia salts are added to solution, with anions of which calcium forms soluble salts: NH4Cl, NH4Br, NH4NO3. Thus ions of ammonium behave here as acid, displacing carbonic acid:
CaCO3 + 2NH4Cl = CaCl2 + 2NH3 + H2O + CO2
Unlike Ве(Mg)СО3, the solubility of СаСО3 is not changed in presence of the carbonates of alkali metals, it means that it does not form double carbonates.
However, СаСО3 in the presence of carbonic acid the excess is transformed easily into well water-soluble hydrocarbonates:
СаСО3 + СО2 + Н2О = Са(НСО3)2
This is a continious process in nature, converting the insoluble limestone into soluble acidic salts which are present in natural water and predetermine its temporal (carbonate) hardness.
Hardness of water and its removal
Sulfates of alkaline-earth metals are white crystalline hardly soluble compounds, whose solubility the series of CaSO4 SrSO4 BaSO4 RaSO4 falls quickly. The insolubility of BaSO4 serves for sulfates content quantitative measurements. Salts SrSO4 and BaSO4 crystallize without water, and СаSO4 at temperatures > 66C is deposited from solution in the form of water-free anhydrite СаSO4, and at temperatures < 66oC as dihydrate or gypsum СаSO4∙2H2O. In nature gypsum is encountered in large beds and finds wide application.
At heating gypsum to 170С a greater part (but not all) of hydrated water is removed from its composition transforming gypsum into CaSO4∙0.5H2O known as alabaster (plaster of Paris). If it is mixed again into a dough with water content 60-80%, there occurs hardening of all mass due to crystallization of dihydrates. As such hardening causes a slightly increased volume, good molds are therefore received as an imprint of objects.
BaSO4 has enormous stability in relation to air, elevated temperatures, action of different harmful gases (especially H2S), it is not toxic for a man. Therefore it is applied as an addititive to the mineral white paints and contrast substance in x-ray analysis.
Nitrates are obtained by interaction of carbonate with a technical nitric acid:
MeCO3 + 2HNO3 = Me(NO3)2 + CO2 + H2O (Ca, Sr, Ba)
This reaction of Ca(NO3)2 (Norwegian saltpetre) leads to nitrogen-calcium fertilizer production.
At heating, nitrates of Ca, Sr and Ba are decomposed:
2 Me(NO3)2 2MeO + 4NO2 + O2
Phosphates of Ca are mineral fertilizers (million tons of annual output). Grinded Ca3(PO4)2 as a phosphorite flour is produced from natural phosphorites, CaHPO4∙2H2O (precipitate) and Ca(H2PO4)2 (super phosphate) are prepared artificially.
