
- •Electronic Configurations & Oxidation States
- •Physical properties
- •Physical Properties. Summary Some Alkaline-Earth Metals Subgroup Trends
- •History Of Discovery
- •Preparation
- •In industry:
- •Alkaline-earth metals chemical properties (1)
- •Alkaline-earth metals chemical properties (2)
- •Alkaline-earth metals chemical properties (3) Compounds hydrides
- •Tests for alkali and alkaline-earth metals subgroups elements
- •Hardness of water and its removal
Electronic Configurations & Oxidation States
Akaline-earth metals subgroup elements are s-elements with two electrons at the external energy level, n:
ns2,
Therefore ns2-electrons are easily removed and Met+2 state is energetically favourable. Alkaline-earth metals always have the oxidation state +2 in compounds.
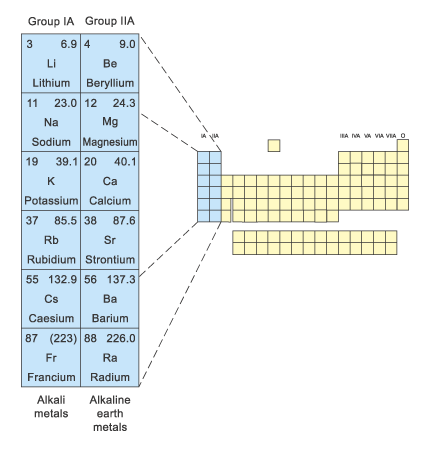
The name alkaline-earth elements are attributed to those metals whose oxides (“earths” of alchemists) produce an alkaline reaction in water.
Ca, Sr, Ba and Ra form a series of elements, within which their chemical and physical properties change regularly, similarly to the s-elements of the first group behavior. The examples of such systematic variation of properties in the Ca—Sr—Ba—Ra series are:
diminishment of solubility of sulfates, nitrates, chlorides and others;
increase of thermal stability of carbonates, nitrates, peroxides;
increase of reaction rates of those metals interaction with hydrogen.
Like Fr, all isotopes of Ra are radioactive. Most long-living isotopes 226Ra have the half-decay period Т1/2 1600 years.
Physical properties
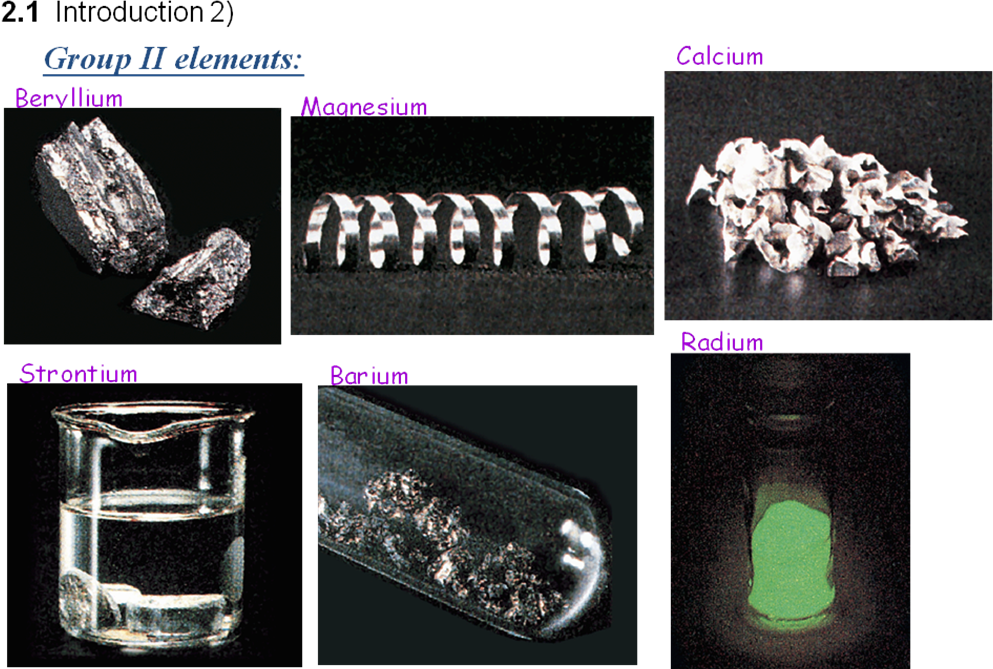
Be and Mg are lustrous metals that are quickly covered by thin coating layer of oxides, which gives them opaque appearance. Be is a metal of light-gray color, it is hard and fragile, and Mg is silverish-white, considerably more soft and more plastic metal. Mechanical strength of Be considerably exceeds mechanical strength of other metals and their alloys, but due to large fragility its application as a construction metal is complicated and limited.
Be (unlike many metals) is diamagnetic, while Mg is a paramagnetic material. Beryllium transparency to X-rays is its unique property; it is used for making windows in X-ray emission tubes.
At fresh cut Ca, Sr, Ba and Ra are silvery white metals, whose fresh cut dim on air quickly as a result of interaction with О2 and N2.
According to physical properties they stand nearer to the alkali metals, than Mg, which stands at the top of the group:
they are considerably softer than Mg;
they also should be kept under the layer of kerosene;
Ca, Sr, Ba, Ra are easily oxidized at the surface, but are heavier, than alkali metals;
they self-ignite easily (Ва ignites on air already at its squashing).
Three allotropes of cаlcium are known. They can be prepared by heating of the lower temperature allotrope named α-Ca. Alkaline-earth elements in nature contain a mixture of stable isotopes (only Ве is solely 9Be).
Compounds of alkaline-earth metals impart specific colours to the flame: Са gives orange-red color, Sr and Ra is carmine-red, Ba is yellowish-green.
Physical Properties. Summary Some Alkaline-Earth Metals Subgroup Trends
|
Be
|
Mg |
Ca |
Sr |
Ba |
Electronic configuration |
[He]2s2 |
[Ne]3s2 |
[Ar] 4s2 |
[Kr]5s2 |
[Xe]6s2 |
Atomic radius, nm |
113 |
160 |
197 |
215 |
221 |
Ionic radius М2+, nm |
34 |
74 |
104 |
120 |
138 |
Potential of ionization, eV I1 : M0 М+ + e |
9.30 |
7.65 |
6.10 |
5.70 |
5.21 |
Electronegativity |
1.5 |
1.2 |
1.0 |
1.0 |
0.9 |
Melting point, С |
1283 |
650 |
847 |
770 |
718 |
Density, g/cm3 |
1.85 |
1.74 |
1.54 |
2.63 |
3.76 |
Hydration heat, H298, kJ/mol |
-2456 |
-1954 |
-1615 |
-1477 |
-1339 |
Ео М2+/М0, V |
-1.847 |
-2.363 |
-2.866 |
-2.888 |
-2.906 |
Conductivity (Hg = 1) |
23 |
22 |
22 |
4 |
2 |
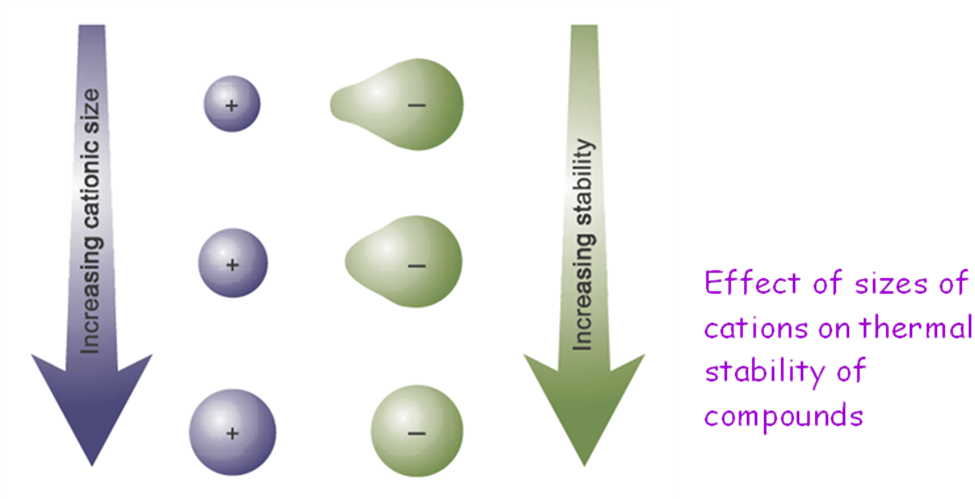
Melting points of Groups I and II elements are the function of metallic bonds strength. The stronger is the bond, the higher is the melting point.
Metallic bond strength depends on: (1) ionic radius,
 (2) number
of valence electrons
(2) number
of valence electrons
Down the group, the size of cations increases
Þ polarizing power decreases
Þ compound with large anion becomes more stable
∴ thermal stability of carbonates & hydroxides of Group I metals increases down the group
