- •Inorganic chemistry
- •0514 Branch “Ecology”
- •Inorganic chemistry……..
- •Introduction
- •Lecture 1. Hydrogen
- •History of discovery
- •N 1 in periodic system
- •Occurrence
- •Physical properties
- •Industrial production of hydrogen
- •1. Iron-steam method
- •Chemical properties
- •Classification of hydrides
- •Lecture 2. Oxygen
- •Physical properties
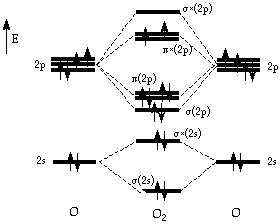
- •Selected properties of the oxygen atom and molecule
- •History of discovery
- •A. Lavoisier
- •Oxygen occurrence
- •Oxygen preparation
- •Preparation in laboratory
- •5. Electrolysis of water
- •Oxygen as a ligand
Chemical properties
The summary of chemical properties of H is shown below:
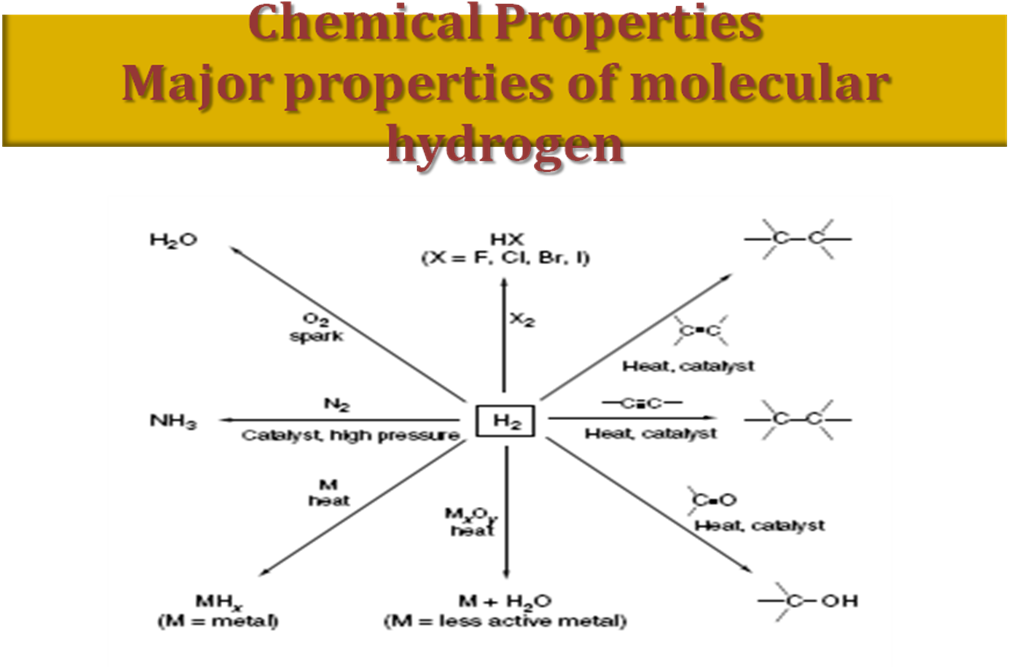
Fig. 1.2. Major properties of molecular hydrogen
The electronegativity of hydrogen is 2.1. It means that it has oxidation state +1 in compounds with typical non-metals, and oxidation state –1 in compounds with metals, respectively. Therefore, Н2 can behave both as oxidant and as reductant. In the reactions with active metals Н2 is oxidant when heated:
Ca + H2 = CaH2
But an oxidizing behaviour is not characteristic of hydrogen.
Saline hydrides are quickly decomposed by water due to their very strong reductive properties ( Eo= –2,34 V):
LiH + Н2О = LiОH + Н2
Classification of hydrides
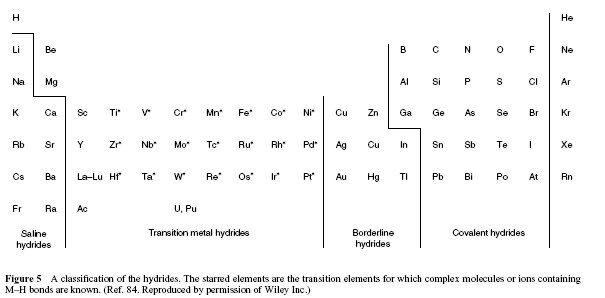
*The starred are the transition elements the hydrides (ionic, complex compounds with M-H bonds) of which are known
Ionic (saline) hydrides are colourless crystalline solids that either melt or decompose at temperatures above 600 ◦C. All of the hydrides can be formed by direct combination of the elements at elevated temperatures. For lithium and sodium the hydrides can also be prepared by heating the corresponding nitride in a stream of hydrogen:
2M3N + 3H2 =6MH + N2
All molecular hydrides other than alkanes and water are poisonous gases with very high reactivity and should be handled very carefully:
H2S, HF, NH3 ammonia ,PH3 phosphine, SiH4 silane, etc.
Metallic hydrides MHx displaying metallic properties are nonstoichiometric interstitial-type solids with hydrogen that occupies a part of cavities in the lattice of a metal.
Palladium, for instance, reacts with hydrogen gas at ambient temperature, and forms hydrides having the composition PdHx(x<1).
Reducing properties of hydrogen are more characteristic.
Molecular Н2 is not so active, it explosively reacts only with F2 under any conditions (even at the temperature of liquid nitrogen, 77 K, hydrogen reacts violently with fluorine):
H2 + F2 = 2HF
The reaction with oxygen is exothermic.
2H2 + O2 =2H2O
The heat required to produce gaseous and liquid H2O in this reaction (25◦C and 1atm) is 242 and 286 kJ mol−1, respectively. This reaction proceeds spontaneously at above 500 ◦C. At lower temperatures this reaction occurs in the presence of catalysts, such as Pt or Pd, or if activated by an electric spark or a flame. The flame temperature at combustion of hydrogen in oxygen atmosphere achieves 2800 ◦C (the technique is applied in oxyhydrogen welding torches). When exposed to sunlight, hydrogen combines with chlorine to form HCl. Hydrogen also reacts with Br2, S, I2, Se and Te. It reacts with N2 and gives ammonia (Haber-Bosch process) when heated, under high pressure, and in the presence of pyrophoric iron catalyst.
Reducing properties of molecular Н2 are expressed weakly in aqueous solutions.
The reduction of metal oxides (MxOy ) by hydrogen gas is metallurgically valuable. Water is produced in this reaction:
![]()
Only a limited number of compounds are reduced by H2 at room temperature. Among them, aqueous solution of palladium chloride that is readily reduced:
PdCl2(aq) + H2 = Pd(s) + 2HCl(aq)
Reducing properties of atomic hydrogen are expressed considerably stronger. Under ordinary conditions it reduces such oxides as CuO, PbO, HgO, Ag2O, Bi2O3. Atomic Н can exist for a very short period of time (0.3 — 0.5 sec.), but sufficient to realize a more active interaction.
So, the addition of metallic Zn into acid solution of KMnO4 is accompanied by discoloration of MnO4- as a result of its reduction when hydrogen at the moment of liberation is formed. At the same time, passing molecular H2 through the acidic solution of KMnO4 makes no change of solution color.
Water H2O. H2O is the most abundant in nature compound of hydrogen. It covers 3/4 of the surface of Earth. Water is a very important technological substance. For example, for obtaining 1 t of iron 300 t of water are used. The general consumption of pure water in Ukraine is approximately 0.01 km3.
Physical properties. Conductivity of water depends on its purity. Conductivity of distilled water equals (80-100).10-6 Сm/m as a result of СО2 absorption from air and Н+ and НСО3- ions formation.
Water has an extraordinarily high heat of melting. To melt 1 kg of ice 332.4 kJ of energy are necessary (by 2 (15) times more than to melt 1 kg of steel or Pb, respectively). It is this property of water that accounts for slow heat of the Earth surface in spring preventing catastrophic disasters.
Water has an extraordinarily high value of heat of evaporation (amount of heat necessary for 1 g of substance evaporation). More than 2000 kJ of energy is required to evaporate 1 L of water. It is the enormous value of heat of evaporation that explains the high temperature of water boiling (100 оС) when the pressure of water steam achieves atmospheric pressure.
More moderate climate is typical of the shores areas of seas and oceans. In summer the seas reduce heat because water is slowly heated and a lot of heat goes for its evaporation. By the winter an atmosphere is heated at freezing of large the masses of water, and especially, when water crystallizes in spacious air (a snow).
Water is an outstanding solvent. The polar molecules of gases are well soluble in water. Solubility of gases increases with the growth of their pressure and vice versa at temperature rise.
Nonpolar gases are low-soluble in water. Polar liquids are well dissolved in water.
Water is the most frequently used medium to realize chemical transformations.
Chemical properties. Water is quite stable at heating. It has no decomposition up to 1200 оС and only dissociates thermally to Н atoms and molecular particle OH at higher temperatures. At 5000 оС it decomposes with explosion.
The most characteristic are the following chemical reactions of water:
1. Combination reaction with basic and acid oxides (formation of bases and acids):
Li2O + H2O = 2LiOH
SO3 + H2O = H2SO4
2. Ability to build up crystal lattice together with bases, acids and salts with the formation of crystalhydrates: КОНН2О, H2SO42Н2О, CuSO45Н2О (blue vitriol), Na2SO410Н2О.
3. Oxidizing properties due to hydrogen oxidation state (+1):
2Na+ 2H2O = 2NaOH + H2
C + H2O = CO + H2
CO + H2O = CO2 + H2
4. Reducing properties due to oxygen oxidation state (-2):
2F2 + 2H2O = 4HF + O2
5. Donor-acceptor interactions. Unshared pairs of electrons of oxygen atom explain reactions of coordination compound formation, with water molecule being ligand:
Cu2+ + 4 Н2О = [Cu(Н2О)4]2+
6. Water is not only a medium. It also can display redox properties:
Cl2 + Н2О = HCl + HOCl
2KMnO4 + 3K2SO3 + Н2О = 2MnO2 + 3K2SO4 + 2KOH
2Cr3+ + 3S2O82- + 7Н2О = Cr2O72- + 6SO42- + 14H+
Heavy water. It is the compound D2O (oxide of heavy hydrogen). It was first obtained in 1933 by electrolysis of alkaline solution. The content of it in salt water makes up approximately 0.018%, therefore to get 1 kg of this compound it is required to decompose approximately 170 t of ordinary water.
Reduction of hydrogen by carbon. Lavoisier passed Н2O through the muzzle of a gun incandescent to red. This method was a long time the main industrial method of production of hydrogen.
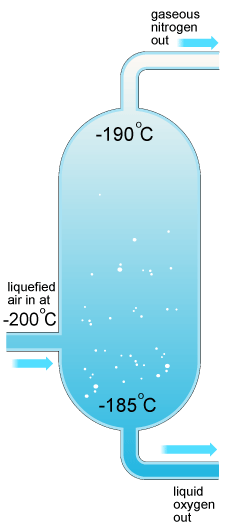
Purification of hydrogen gas to eliminate admixtures of СО2, N2, CO is achieved by cooling the gas mixture in liquid air at –200оС. High purity hydrogen for food industry (for vegetable oil hydrogenation, for margarine production) is obtained by this method.
15.9994 Oxygen Electronic Configuration [He]2s22p4 |
Oxygen |
||||
|
|||||
O (Ox. State) |
Compounds of Oxygen. Chemical and Physical Properties Overview
|
||||
-2 |
H2O, SO2, K2SO3, KOH, H2SO3 |
|
|
||
-1 (O22-) |
H2O2, Na2O2, BaO2 |
|
|
MO representation |
|
-1/2 (O2-) |
KO2, RbO2, CsO2 |
superoxides |
|
|
|
-1/3 (O3-) |
M[O3], M=Na-Cs |
ozonides |
|
|
|
0 |
O2, O3, O4 |
|
|
|
|
+1/2 (O2+) |
O2+[PtF6] |
|
|||
+1 |
O2F2 |
||||
+2 |
OF2 |
||||

 8
8