
- •Тема 1 – Методы химических превращений, используемые в технике получения органических соединений (3 часа)
- •Тема 3 – Technology for obtaining inorganic drugs (3 hours)
- •Тема 4 – Технология получения синтетических лекарственных веществ (3 часа)
- •Тема 5 – Технология получения синтетических лекарственных веществ (3 часа)
- •Методические рекомендации для самостоятельной работы студентов под руководством преподавателя (срсп)
- •Тема 1 - Процессы хлорирования для получения промежуточных продуктов синтеза лекарственных веществ (3 часа)
- •Тема 2 – Значение концентрации серной кислоты и понятие о п-сульфировании (3 часа)
- •Тема 3 – Техника и практика нитрования (3 часа)
- •Тема 4 – Общие понятия о процессах конденсации. Введение кетогруппы по реакции Фриделя-Крафтса (3 часа)
- •Тема 5 – Рубежный контроль (3 часа). Методические рекомендации для самостоятельной работы студентов (срс)
- •Тема 1 – Восстановление. Каталитическое гидрирование (5 часов)
- •Тема 2 – Ацилирование. Ацилирующие средства (5 часов)
- •Тема 3 – Производство солей и их значение в химико-фармацевтической промышленности (5 часов)
С.Ж.АСФЕНДИЯРОВ АТЫНДАҒЫ ҚАЗАҚ ҰЛТТЫҚ МЕДИЦИНА УНИВЕРСИТЕТІ |
|
КАЗАХСКИЙ НАЦИОНАЛЬНЫЙ МЕДИЦИНСКИЙ УНИВЕРСИТЕТ ИМЕНИ С.Д.АСФЕНДИЯРОВА |
MODULE «PHARMACIST - ANALYST»
WORKING PROGRAM
Teaching-methodical complex on elective discipline
«Preparation and investigation of medicinal substances»
3 course
content
Рабочая программа ………………………………………………………………………………………. 3
Силлабус ………………………………………………………………………………………………... 11
Методические рекомендации для занятий
(практических, семинарских, лабораторных) ……………………………………………………….. 20
Методические рекомендации для самостоятельной
работы студентов под руководством преподавателя ………………………………………………... 30
Методические рекомендации
для самостоятельной работы студентов ……………………………………………………………… 39
Контрольно-измерительные средства
для оценки знаний, умений т навыков
по дисциплине ………………………………………………………………………………………….. 46
Approve
Vice-Principal for teaching- practice work
Professor Tulebayev K.A.
__________________
«__»_________2012
Working program
Discipline «Preparation and investigation of medicinal substances»
Specialty 5В051103 – «Pharmacy»
Course 4
Semester VII
Practical lessons - 15 hours
Student’s self-learning under teacher’s supervision - 7 hours
Student’s self-learning - 23 hours
Assessment form: examination (test, oral exam)
Academic hours volume
(credits) 90 hours (2 credits)
Almaty 2012
Working program has been discussed at the «Farmacist-analitik» module sitting dated «____» _____ 2012, protocol № ____.
Head of the module, Doctor of chemical sciences, Professor Omarova R.A.
Working program has been discussed at the sitting of the Committee for degree programs of Pharmacy
dated «____» _____ 2012 , protocol № ____.
Chairman of the Committee, assistant professor Associate (docent) Sayakova G.M.
Working program has been discussed at the sitting of the Methodological Board dated «____» _____ 2012 , protocol № ____
Chairman of the Methodological Board, professor Tulebayev K.A.
1. General information:
Name of the Institution of higher education: Kazakh National Medical University named after Asphendiyarov S.D.
Module: «Pharmacist - analyst»
Specialty: 5В051103– «Pharmacy»
Discipline: Preparation and investigation of drug
Amount of credits: 1 credit, 45 hours
Course and semester of learning: 4th course, 7 semester
2. Program
2.1 Brief description of the discipline:
Each new drug is phased manufacturing process, which begins with obtaining drugs in the laboratory and on the production end model or factory installations.
Getting drugs in the laboratory is the longest stage of the production, including a survey and study of the methods of processing of natural or chemical materials, the study of processes of production of intermediate products and methods of their implementation with the least expenditure of energy and resources.
The study of various methods of processing raw materials into intermediate products is possible by combining processes in a group of similar basic typical reaction processes for fine chemicals. In a series of chemical reactions that accompany the process, the chemical processes are introducing substituents in organic compounds: halogenation, sulfonation and sulfohlorirovanie, nitration and nitrosation. The second group of chemical transformations include conversion processes introduced substituents: recovery, diazotization, acylation, esterification, alkylation, arylation, hydrolysis (hydroxylation), ammonolysis, alkaline melting. The third group includes the processes that change the carbon skeleton of the organic compounds (oxidation, reduction, condensation and rearrangement).
In addition to chemical transformations, the process involves physical treatment methods: filtration, precipitation, evaporation, crystallization, drying, distillation, etc.
Thus, the transformation of the intermediate product in the production of the final product is accompanied by a variety of manufacturing operations. Based on the study of processes at the stage of new drugs are taken of the processes that are the most advanced, cost-effective, associated with the use of available and cheap raw materials and hardware design, easier and less phasic subject to the safe conduct and a minimum loss of production of the final product .
2.2 The objective of discipline:
Bring the theoretical knowledge of students in the practical aspects of obtaining drugs.
2.3 Tasks: Discipline teaching shall envisage:
• consolidate students' knowledge on the theoretical foundations of chemistry and technology of drugs;
• to teach the basics of chemical transformations of intermediate or final products of laboratory production;
• to educate students on the basics of physical methods of processing of raw materials;
• to practice the methods of obtaining drugs in the laboratory.
2.4 Training outcomes:
The student should know:
• the main types of chemical reactions used for the synthesis of drugs
• Physical methods of processing raw materials;
• technology of obtaining inorganic drugs;
• technology of obtaining synthetic drugs;
The student shall be able to:
• master the technological stages of obtaining the product production in the laboratory;
• learn practical techniques for the synthesis of intermediates;
• carry out the synthesis of inorganic and organic drugs.
2.5 Prerequisites:
analytical chemistry, physical chemistry, general methods of research and analysis of drugs, physical and chemical methods of research in pharmacy.
2.6 Postrequisites: pharmaceutical chemistry, toxicological chemistry, pharmacognosy.
2.7 Thematic plan: topics, form of teaching and duration of every lesson (practical lessons, student’s self-learning under a teacher’s supervision, student’s self-learning)
2.7.1 Academic-thematic calendar of practical lessons
№ |
Practical lessons topics |
Lesson’s duration |
1 |
Methods of chemical reactions that are used in the technique of preparation of organic compounds: Work 1 - the oxidation in obtaining benzoic acid; Work 2 - halogenation in obtaining iodoform; Work 3 - hydroxylation reaction in the production of phenol. |
3 |
2 |
substances: Work 1 - receiving barium sulfate for X-rays; Work 2 - Determination of the theoretical and practical output of barium sulfate for X-rays.
|
3 |
3 |
Technology for producing inorganic medicinal substances: Work 1 - receiving sodium sulfate; Work 2 - Determination of the theoretical and practical output of sodium sulfate |
3 |
4 |
Technology for producing synthetic medicinal substances: Work 1 - receiving hexamethylenetetramine; Work 2 - Define the output of the drug. |
3 |
5 |
Technology for producing synthetic medicinal substances: Work 1 - receiving sodium benzoate; Work 2 - Define the output of the drug. |
3 |
Total |
15 hours |
|
2.7.2 Thematic calendar of student’s self-learning under teacher’s supervision
№ |
SSLSUTS topics |
Study duration |
1 |
Chlorination process to produce synthesis intermediates of medicinal substances. |
2 |
2 |
The concentration of sulfuric acid, and the notion of p-sulfonation. |
2 |
3 |
Technique and Practice of nitration. |
1 |
4 |
General concepts of the condensation process. The introduction of a keto group by Friedel-Crafts reaction. |
1 |
5 |
Boundary line of control. |
1 |
Total |
7 |
|
2.7.3. Thematic calendar of student’s self-learning
№ |
Topics of SSL |
Study duration |
1 |
Recovery. Catalytic hydrogenation. |
8 |
2 |
Acylation. Acylating agent. |
8 |
3 |
Production of salt and their importance in the chemical and pharmaceutical industries. |
7 |
Total |
23 |
|
2.8 Methods of teaching and learning (small groups, work in pairs, etc.):
small groups
forms of teaching practical lessons, student’s self-learning under a teacher’s supervision, student’s self-learning
Practical training: Control students on self-study, laboratory work on the subject, the discussion laboratory work, design protocol analysis, test control
Students’ self-tuition under teacher’s guidance – A panel discussion with illustrative tables, presentation material, individual reports, role play.
Students’ self-tuition activity – Work with the literature on issues provided for self-study, preparation of scientific abstracts
2.9 Methods for assessing the knowledge and skills of students: oral questioning and testing. The technology of controlling student's knowledge
I = R х 0,6 + E х 0,4, where
I – final grade
R– rating allowance grade
E – final assessment grade (exam on discipline)
Rating comes to 60% off I
Exam -40% off I
Trainees’ rating grade is formed of current and intermediate assessments grades.
First rating is calculated according to the formula:
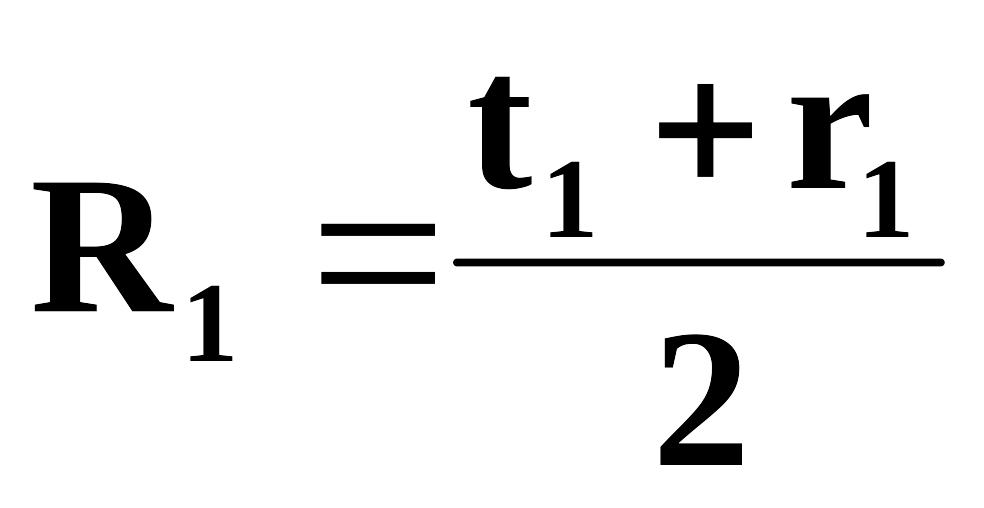 t
– current assessment= average grade for practical training
(laboratory
works, seminars)
+ average
grade for students’ self-tuition work under teacher’s supervision
+ average
grade for students’ self-tuition work
t
– current assessment= average grade for practical training
(laboratory
works, seminars)
+ average
grade for students’ self-tuition work under teacher’s supervision
+ average
grade for students’ self-tuition work
r - intermediate assessment
Every practical lesson, students’ self-tuition work under teacher’s supervision, students’ self-tuition work, intermediate assessment are calculated out of 100 points which corresponds to 100%. Second rating is calculated according to the formula:
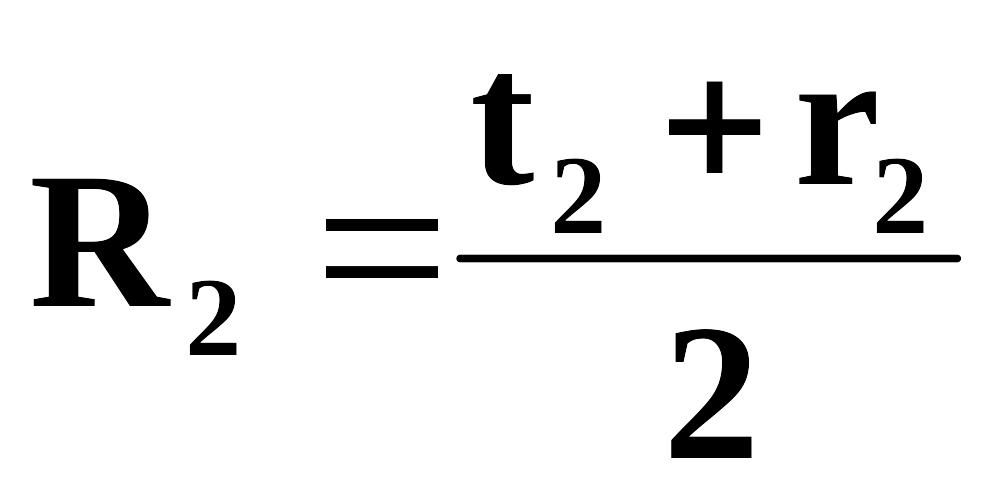
t – current assessment= average grade for practical training (laboratory works, seminars) + average grade for students’ self-tuition work under teacher’s supervision + average grade for students’ self-tuition work, r - intermediate assessment
Allowance rating in the student’s final grade comes to minimum 60 %, therefore the trainees’ semester grade on the discipline is defined according to the formula:
![]() A
trainee is considered to be admitted to an exam if his/her semester
grade is higher or equal to 30%.
A
trainee is considered to be admitted to an exam if his/her semester
grade is higher or equal to 30%.
In case of non-availability of intermediate controls the allowance rating is calculated solely proceeding from current grades.
In case of big number of intermediate assessments there is calculated a corresponding number of ratings, in the end of semester the average rating is calculated.
Technology of conducting and assessing the exam
Maximum percentage content of a final exam corresponds to 100 %. Examiner puts a final control grade (E) into an exam record applying the instruments of students’ knowledge metrology.
Final assessment metrology instrument in the form of testing
At the exam a student is given 50 test tasks, i.e. every task corresponds to 2 points or percents.
№ |
Quality of works execution |
Assessment range |
1 |
Non-fulfilled Absence at the exam without sound reasons |
0%
|
2 |
Grade for every correct answer |
2% |
|
Total: |
0-100% |
Share of final exam grade comes to maximum 40% off the final examination grade on the discipline, therefore examination grade Ex on the discipline is multiplied by a coefficient 0,4 Ex0,4
Further on the final grade is calculated
I = R х 0,6 + E х 0,4
Final assessment: State exam.
2.10. References:
Basic:
1 JG Zielinski etc. Isolation and purification of substances himfarmpromyshlennosti / JG Zelinsky, BV Shemeryankin, NM Shmakov. - M.: Medicine, 1982. - 240. 2 grazes BV, MA Antipov Workshop on technical analysis and control in the production of chemical and pharmaceutical drugs and antibiotics [for chem. - Pharmacy. technical schools]. - M.: Medicine, 1981, 272 p. 3 State Pharmacopoeia of the Republic of Kazakhstan. 1 vol. - Almaty: ed th house "Silk Way", 2008 - 592 p.
Supplementary:
1 Albitskaya VG, Ginsburg OF, Kolyaskina ZN, Kupin BS, LA Pavlova, Razumova NA, Rall KB, Serkova VI Stadnichuk MD Laboratory work in organic chemistry. Ed. OF Ginzburg, AA Petrov. Vysshaya. wk., 1967. - 295 p. 2 Maiofis LS Chemistry and technology of chemical and pharmaceutical products. 2nd ed., Rev. and add. - M.: Medicine, 1964. - 625 p.
2.11.Applications: - Form № 1 "Protocol of teaching from pre-and prerequisites - as required - Form № 2 "Additions and changes to the work program" - as required
Faculty:Pharmacy Module: Pharmacist-аnalyst
Syllabus
for the discipline: Preparation and investigation of medicinal substances (name of discipline)
Курс 3 (очное отделение)

lecture - exam VI

 (semester)
(semester)
 practical
lessons
30 hours
SSL
46
hours
practical
lessons
30 hours
SSL
46
hours
SSLSUTS 14 hours
The volume of training hours (credits) 90 hours/2 credit
Аlmaty 2012 y.
Syllabus for elective courses, "Preparation and investigation of medicinal substances" was developed in accordance with the in 2008 and compiled by the lecturer of module pharmacist - analyst Boshkaeva AK
Syllabus approved at the module pharmacist - analyst
protocol № 16
« 28» 04. 2011 г.
The head of Module pharmacist - analyst
Professor Omarova R.A.
1. General information:
Name of the university: S.D. Asfendiyarov Kazakh National Medical University.
Module: «Pharmacist - analyst»
Discipline, discipline code: Preparation and investigation of medicinal substances
Special: 051103 – Pharmacy
The volume of hours / credits: 90 hours / 2 credits
The course and semester of study: 3 year, VI semester
Information about the teacher:
Boshkaeva Asyl Kenesovna, Candidate of Pharmaceutical Sciences, Associate Analyst module pharmacist. -Research Interests: physico-chemical studies of biologically active compounds, general chemistry (molecular structure, the atomic nucleus), electronic submission in organic chemistry.
1.8 Contact Information: Department of the location (address, building, room), phone, e-mail
1.9 Discipline Policy: General Requirements Department applicable to students in the study of the discipline, punitive measures in case of non-compliance with sections etc..
1. Mandatory, regular attendance of practical lessons, student’s self-learning under teacher’s supervision and SSL;
2. Active participation in the learning process (synopsis of practical work, training of theoretical material, the solution tests, independent of case work).
3. Filing reports of practical work, tests, topics CDS protection at a set time on a thematic plan no later than the week corresponding to this section;
4. The presence of an individual student's diary to record all the activities.
2. program:
2.1 Introduction:
Each new drug is passed manufacturing process, which begins with obtaining drugs in the laboratory and on the production end model or factor installations.
Getting drugs in the laboratory is the longest stage of the production, including a survey and study of the methods of processing of natural or chemical materials, the study of processes of production of intermediate products and methods of their implementation with the least expenditure of energy and resources.
The study of various methods of processing raw materials into intermediate products is possible by combining processes in a group of similar basic typical reaction processes of fine chemicals. In a series of chemical reactions that accompany the process, the chemical processes are introducing substituents in organic compounds, halogenation, sulfonation and sulfohlorirovanie, nitration and nitrosation. The second group of chemical transformations include conversion processes introduced substituents: recovery, diazotization, acylation, esterification, alkylation, arylation, hydrolysis (hydroxylation), ammonolysis, alkaline melting. The third group includes the processes that change the carbon skeleton of the organic compounds (oxidation, reduction, condensation and rearrangement).
In addition to chemical transformations, the process involves physical treatment methods: filtration, precipitation, evaporation, crystallization, drying, distillation, etc. Thus, the transformation of the intermediate product in the production of the final product is accompanied by a variety of manufacturing operations. Based on the study of processes at the stage of new drugs are taken of the processes that are the most advanced, cost-effective, associated with the use of available and cheap raw materials and hardware design, easier and less phasic subject to the safe conduct and a minimum loss of production of the final product .
2.2 The purpose of discipline: Bring the theoretical knowledge of students in the practical aspects of obtaining drugs.
2.3 Learning objectives: • consolidate students' knowledge on the theoretical foundations of chemistry and technology of drugs; • to teach the basics of chemical transformations of intermediate or final products of laboratory production; • to educate students on the basics of physical methods of processing of raw materials; • to practice the methods of obtaining drugs in the laboratory.
2.4 Learning outcomes:
The student should know:
• the main types of chemical reactions used for the synthesis of drugs; • technology of inorganic chemical and pharmaceutical products; • mass spectrometry; • GC-MS methods.
The student should be able to:
• carry out structural studies of drug with physico-chemical methods;
• to interpret the results of physico-chemical analysis of drugs;
• evaluate the effectiveness of the obtained spectral investigations.
2.5 Prerequisites: analytical chemistry, physical chemistry, general methods of research and analysis of drugs, physical and chemical methods of research in pharmacy.
2.6 postrequisites: pharmaceutical chemistry, toxicological chemistry, pharmacognosy.
2.7 Summary of the discipline:
Sources for drugs is the different materials. mineral materials are the sources of inorganic drugs. For the production of organic drugs use dry distillation of wood, oil shale, coal, and various petroleum fractions.
from plant materials releases essential and fatty oils, resins, proteins, and carbohydrates. plant materials are natural source of biologically active substances, alkaloids, terpenes, glycosides, and vitamins.
From raw materials of animal origin (organs, tissues, glands beef cattle), get individual substances - hormones.
From marine organisms (algae, cod liver oil), get some drugs.
Technology of producing medicinal preparation covers all stages of the of intermediates and final products. Study of technological processes of production of intermediates and drug substances and methods of their implementation with the least expenditure of raw materials, research and study of methods for the processing of natural and chemical raw materials in drugs and choose the most cost-effective and improved methods of obtaining an integral part of the content of the discipline.
2.8 Thematic plan: themes, form and duration of each session (practical lessons, student’s self-learning under teacher’s supervision, student’s self-learning).
thematic plan of practical lessons
№ п/п |
The topics |
Lesson’s duration |
1. |
Methods of chemical reactions that are used in the technique of preparation of organic compounds: Work 1 - the oxidation in obtaining benzoic acid; Work 2 - halogenation in obtaining iodoform; Work 3 - hydroxylation reaction in the production of phenol. |
3 hours |
2. |
Technology for producing inorganic medicinal substances: Work 1 - receiving barium sulfate for X-rays; Work 2 - Determination of the theoretical and practical output of barium sulfate for X-rays.
|
3 hours |
3. |
Technology for producing inorganic medicinal substances: Work 1 - receiving sodium sulfate; Work 2 - Determination of the theoretical and practical output of sodium sulfate |
3 hours |
4. |
Technology for producing synthetic medicinal substances: Work 1 - receiving hexamethylenetetramine; Work 2 - Define the output of the drug. |
3 hours |
5. |
Technology for producing synthetic medicinal substances: Work 1 - receiving sodium benzoate; Work 2 - Define the output of the drug. |
3 hours |
|
Total |
15 hours |
Thematic plan of student’s self-learning under teacher’s supervision
№ п/п |
The topics |
Lesson’s duration |
1. |
Chlorination process to produce synthesis intermediates of medicinal substances. |
2 hours |
2. |
The concentration of sulfuric acid, and the notion of p-sulfonation. |
2 hours |
3. |
Technique and Practice of nitration. |
1 hour |
4. |
General concepts of the condensation process. The introduction of a keto group by Friedel-Crafts reaction. |
1 hour |
5. |
Boundary line of control. |
1 hour |
|
Total |
7 hours |
Thematic plan of student’s self-learning
№ п/п |
Тема |
Lesson’s duration |
1. |
Topics of SSL |
8 hours |
2. |
Recovery. Catalytic hydrogenation. |
8 hours |
3. |
Acylation. Acylating agent. |
7 hours |
|
ИТОГО: |
23 hours |
Time of consultation - on schedule
Consultations be conducted by teachers before two days exam
Еhe time of Boundary line of control – 15 week
Time of final control - on schedule
2.9 References:
Basic:
1 Зелинский Ю.Г. и др. Выделение и очистка веществ в химфармпромышленности / Ю.Г. Зелинский, Б.В. Шемерянкин, Н.М. Шмаков. – М.: Медицина, 1982. – 240 с.
2 Пасет Б.В., Антипов М.А. Практикум по техническому анализу и контролю в производстве химико-фармацевтических препаратов и антибиотиков: [Для хим. – фармац. техникумов]. – М.: Медицина, 1981, 272 с.
3 Государственная фармакопея Республики Казахстан. 1 том. – Алматы: изд-й дом «Жибек жолы», 2008 - 592 с.
Supplementary:
Альбицкая В.Г., Гинзбург О.Ф., Коляскина З.Н., Купин Б.С., Павлова Л.А., Разумова Н.А., Ралль К.Б., Серкова В.И., Стадничук М.Д. Лабораторные работы по органической химии. Под ред. О.Ф. Гинзбурга, А.А. Петрова. М.: Высш. шк., 1967. – 295 с.
Майофис Л.С. Химия и технология химико-фармацевтических препаратов. 2-е изд., перераб. и доп. – М.: Медицина, 1964. – 625 с.
2.10 Methods of teaching and learning (small groups, work in pairs, etc.):
small groups
forms of teaching practical lessons, student’s self-learning under a teacher’s supervision, student’s self-learning
Practical training: Control students on self-study, laboratory work on the subject, the discussion laboratory work, design protocol analysis, test control
Students’ self-tuition under teacher’s guidance – A panel discussion with illustrative tables, presentation material, individual reports, role play.
Students’ self-tuition activity – Work with the literature on issues provided for self-study, preparation of scientific abstracts
2.11 Criteria and rules for assessment of knowledge: the scale and criteria for evaluation of knowledge at every level (current, mid-term, final control), rules for assessing all types of activities (classroom, SSLTS, SSL *)
Methods of trainees’ knowledge and skills assessment
I = R х 0,6 + E х 0,4, where
I – final grade
R– rating allowance grade
E – final assessment grade (exam on discipline)
Rating comes to 60% off I
Exam -40% off I
Trainees’ rating grade is formed of current and intermediate assessments grades.
First rating is calculated according to the formula:
t – current assessment= average grade for practical training (laboratory works, seminars) + average grade for students’ self-tuition work under teacher’s supervision + average grade for students’ self-tuition work
r - intermediate assessment
Every practical lesson, students’ self-tuition work under teacher’s supervision, students’ self-tuition work, intermediate assessment are calculated out of 100 points which corresponds to 100%. Second rating is calculated according to the formula:
t – current assessment= average grade for practical training (laboratory works, seminars) + average grade for students’ self-tuition work under teacher’s supervision + average grade for students’ self-tuition work, r - intermediate assessment
Allowance rating in the student’s final grade comes to minimum 60 %, therefore the trainees’ semester grade on the discipline is defined according to the formula:
![]()
A trainee is considered to be admitted to an exam if his/her semester grade is higher or equal to 30%.
In case of non-availability of intermediate controls the allowance rating is calculated solely proceeding from current grades.
In case of big number of intermediate assessments there is calculated a corresponding number of ratings, in the end of semester the average rating is calculated.
Technology of conducting and assessing the exam
Maximum percentage content of a final exam corresponds to 100 %. Examiner puts a final control grade (E) into an exam record applying the instruments of students’ knowledge metrology.
Final control measurement tool for oral examination (examination by tickets in the ticket 3 questions)
№ |
Quality of execution work |
Assessment range |
1 |
not done The absence of an exam without a valid reason |
0%
|
2 |
Evaluation of knowledge on each issue |
0-30% |
3 |
Evaluation of additional issues |
0-10% |
|
Total: |
0-100% |
Final assessment metrology instrument in the form of testing
At the exam a student is given 50 test tasks, i.e. every task corresponds to 2 points or percents.
№ |
Quality of works execution |
Assessment range |
1 |
Non-fulfilled Absence at the exam without sound reasons |
0%
|
2 |
Grade for every correct answer |
2% |
|
Total: |
0-100% |
Share of final exam grade comes to maximum 40% off the final examination grade on the discipline, therefore examination grade Ex on the discipline is multiplied by a coefficient 0,4 Ex0,4
Further on the final grade is calculated
I = R х 0,6 + E х 0,4
Final assessment: State exam.
In the examination sheets exhibited the final assessment of the discipline in the number and letter equivalent points according to the following table.
Score literal system |
Digital equivalent points |
Percentage % |
Score from the traditional system |
А |
4,0 |
95-100 |
EXCELLENT |
А- |
3,67 |
90-94 |
|
В+ |
3,33 |
85-89 |
GOOD |
В |
3,0 |
80-84 |
|
В- |
2,67 |
75-79 |
|
С+ |
2,33 |
70-74 |
SATISFACTORILY |
С |
2,0 |
65-69 |
|
С- |
1,67 |
60-64 |
|
D+ |
1,33 |
55-59 |
|
D |
1,0 |
50-54 |
|
F |
0 |
0-49 |
UNSATISFACTORILY |
methodical RECOMMENDATIONS
For practical lessons
Тема 1 – Методы химических превращений, используемые в технике получения органических соединений (3 часа)
Цель: Закрепить знания обучающихся основами химических превращений промежуточных или конечных продуктов производства.
Задачи обучения: разобрать основные типы химических реакции, используемые для синтеза лекарственных веществ и применить в технике получения органических соединений.
Основные вопросы темы:
Реакции замещения в ароматическом ряду.
Процессы галогенирования. Сульфирование, нитрование, нитрозирование, гидроксилирование.
Восстановление, окисление, ацилирование, алкилирование.
Методы обучения и преподавания: разбор теоретических вопросов и выполнение практических заданий.
Литература:
Альбицкая В.Г., Гинзбург О.Ф., Коляскина З.Н., Купин Б.С., Павлова Л.А., Разумова Н.А., Ралль К.Б., Серкова В.И., Стадничук М.Д. Лабораторные работы по органической химии. Под ред. О.Ф. Гинзбурга, А.А. Петрова. М.: Высш. шк., 1967. – 295 с.
Майофис Л.С. Химия и технология химико-фармацевтических препаратов. 2-е изд., перераб. и доп. – М.: Медицина, 1964. – 625 с.
Контроль
Какие реакции замещения используются в практике получения лекарственных веществ?
Приведите конкретные примеры использования хлорпроизводных в синтезе промежуточных продуктов синтеза лекарственных веществ.
Охарактеризуйте понятие о п-сульфировании?
Какие ацилирующие средства используются в процессе ацилирования лекарственных веществ? Оцените природу использования ацилирующих агентов.
5.Какие реакции используются в процессе получения получении йодоформа, фенола, бензойной кислоты?
ПРИЛОЖЕНИЕ
Лабораторная работа 1 - Получение бензойной кислоты.
Толуол 10 г
Перманганат калия 34 г
Соляная кислота
Круглодонная колба на 1 л
Водяной холодильник
Колба Бунзена
Воронка Бюхнера
Стакан
Баня песчаная
Методика. В литровой колбе. Снабженной обратным холодильником, кипятят в течение 4 ч на песчаной бане 10 г толуола с 700 мл воды и 34 г мелкорастертого перманганата калия. Для равномерного кипения реакционной смеси в колбу бросают несколько кусочков пористого кирпича. После окончания реакции бесцветный* раствор охлаждают, выпавшую двуокись марганца отфильтровывают и дважды промывают кипящей водой (по 10-15 мл).
Фильтрат упаривают до объема 100-150 мл и подкисляют концентрированной соляной кислотой до кислой реакции по конго. При этом осаждается бензойная кислоты. ЕЕ отфильтровывают, промывают небольшим количеством холодной воды и сушат. Т.пл. 120-1210. Выход 70-80 % от теоретического [1].
[1]. Альбицкая В.Г., Гинзбург О.Ф., Коляскина З.Н., Купин Б.С., Павлова Л.А., Разумова Н.А., Ралль К.Б., Серкова В.И., Стадничук М.Д. Лабораторные работы по органической химии. Под ред. О.Ф. Гинзбурга, А.А. Петрова. М.: Высш. шк., 1967. – с. 139.
* Если реакционная смесь остается окрашенной, обесцвечивания достигают прибавлением нескольких капель спирта.
Реакции окисления используются для получения кислородсодержащих соединений. Окисление можно проводить кислородом воздуха и различными окислителями. Наиболее часто применяемым окислителем органических соединений является перманганат калия, окислительная способность которого зависит от среды. Для окисления применяют водные растворы перманганата калия различной концентрации в нейтральной, кислой или щелочной средах.
Лабораторная работа 2 – Использование реакции галогенирования при получении йодоформа.
Йодоформ – твердое кристаллическое вещество лимонно-желтого цвета с неприятным запахом. Порог ощущения его запаха 0,000006-0,00027 мг/л, летуч с водяным паром. Температура плавления 116-120 0; удельная масса 4,003. В этиловом спирте при температуре 17-18 0 йодоформ растворяется 1:75, а в эфире 1:10. Хорошо растворим в ацетоне, мало – в бензине.
Производство йодоформа из ацетона сводится к двум химическим стадиям: получению раствора гипохлорита натрия и получению йодоформа с применением операций отстаивания, разделения, декантации, центрифугирования, промывки водой, спиртом и сушки.
Хлоратор из нержавеющей стали или эмалированный. Лучше из антихлор- или монельметалла, снабженный мешалкой, барботером и рубашкой, наполняют 30 %-ным раствором едкого натра. Хлораторы могут быть соединены попарно. В первый пропускают из баллона газообразный хлор через барботер, а второй служит абсорбером для непоглощенного хлора. Процесс идет с выделением тепла. Аппарат охлаждают, не допуская подъема температуры выше 30 0. По окончании хлорирования в первом аппарате хлор пропускают во второй, а первый используют как абсорбер, заливая в него предварительно раствор едкого натра. Полученный раствор гипохлорита натрия доводят водой до удельной массы 1,21-1,27.
Получение йодоформа ведут в аппарате из нержавеющей стали, снабженном рассольным охлаждением. Туда подают раствор гипохлорита натрия и ацетон, а из мерника предварительно загружают раствор натрия или калий йодида. В начале процесса появляется интенсивно желто-коричневое окрашивание, которое может наблюдаться и при избытке гипохлорита натрия. Это обусловлено выделением свободного йода, что совершенно нежелательно, так как ведет к получению, помимо йодоформа, и йодатов (переходящих впоследствии в маточный раствор, снижающих выход йодоформа и приводящих к непроизводительным затратам дорогостоящего натрий йодида).
Рекомендуется вести загрузку реагирующих веществ следующим образом. Растворы гипохлорита и ацетона подают в реактор равномерно и одновременно, и процесс ведут с тщательным перемешиванием и охлаждением аппарата рассолом. Температура реакционной смеси не должна превышать 10 0. Все время наблюдают за реакционной смесью и регулируют подачу растворов так, чтобы окраска была интенсивно-желтой, а не коричневой.
Для определения конца реакции в фильтрат реакционной массы, разлитой в две пробирки, добавляют: в одну пробирку 10-15 капель раствора гипохлорита натрия, а в другую – 3-5 капель ацетона. В случае невыпадения осадка йодоформа в обеих пробирках реакцию считают законченной. Эту пробу повторяют через час, а затем после 30 мин отстаивания маточник сифонируют через ткань и спускают и канализацию. Технический йодоформ после отстаивания, разделения и декантации засасывают в специальный отстойник – аппарат с мешалкой, где промывают 3-4 раза очищенной водой, а затем – раствором хлороводородной кислоты для удаления солей кальция и железа, после чего вновь промывают очищенной водой и передают на центрифугу, где снова промывают водой, затем спиртом. Влажный йодоформ сушат на противнях в калориферной сушилке при 50 0. Сухой продукт просеивают на электровибрационном сите. Фасуют препарат в банки оранжевого стекла. Отсевы подвергают размолу в фарфоровой шаровой мельнице [2].
[2]. Майофис Л.С. Химия и технология химико-фармацевтических препаратов. 2-е изд., перераб. и доп. – М.: Медицина, 1964. – с. 221-223.
Лабораторная работа 3 – Использование реакции гидроксилирования в производстве фенола.
Анилин 9,3 г
Серная кислота (d=1,84) 10 мл
Нитрит натрия 7,3 г
Эфир 60 мл
Стакан фарфоровый на 250 мл
Круглодонная колба на 500 мл
Воронка капельная
Мешалка
Установка для перегонки с водяным паром
Установка для перегонки фенола с коротким воздушным холодильником
В толстостенный стакан наливают 50 мл воды, при тщательном перемешивании осторожно приливают 190 мл концентрированной серной кислоты и добавляют 9,3 г свежеперегнанного анилина. Раствор охлаждают до 0 0, постепенно добавляя к нему 75 г мелко раздробленного льда при сильном перемешивании, чтобы частично выделяющийся сернокислый анилин был мелкокристаллическим.
К охлажденной смеси из капельной воронки медленно, по каплям, при сильном перемешивании приливают охлажденный до 0 - 50 раствор 7,3 г нитрита натрия в 30 мл воды. Температура реакционной смеси при этом не должна превышать 8 0. Когда основная часть раствора нитрита натрия уже находится в реакционной смеси, прекращают его приливание и через 5 мин берут пробу на присутствие свободной азотистой кислоты. Для этого каплю раствора наносят на иодкрахмальную бумагу. Если на бумаге не появляется синее пятно, значит, нужно продолжать добавление раствора нитрита натрия. Одновременно надо следить за тем, чтобы раствор все время имел кислую реакцию (по конго). Наряду с присутствием свободной азотистой кислоты в растворе признаком конца реакции является полный переход сернокислого анилина в раствор.
Раствор соли диазония переносят в колбу на 500 мл и нагревают на водяной бане (температура бани 50-60 0) до прекращения выделения азота (15-20 мин). Полученный фенол отгоняют с водяным паром до тех пор, пока проба дистиллята не перестанет давать помутнения с бромной водой. Дистиллят насыщают поваренной солью и фенол несколько раз извлекают эфиром (3 раза порциями по 20 мл). Эфирные вытяжки сушат безводным сульфатом натрия или магния. Эфир отгоняют и фенол перегоняют из маленькой колбы, собирая фракцию 179-183 0. После охлаждения фенол должен закристаллизоваться.
Выход фенола около 6 г. Фенол вызывает при попадании на кожу ожоги. Поэтому при работе с ним следует соблюдать осторожность [3].
[3]. Альбицкая В.Г., Гинзбург О.Ф., Коляскина З.Н., Купин Б.С., Павлова Л.А., Разумова Н.А., Ралль К.Б., Серкова В.И., Стадничук М.Д. Лабораторные работы по органической химии. Под ред. О.Ф. Гинзбурга, А.А. Петрова. М.: Высш. шк., 1967. – с. 109-110.
Topic 2 – Technology for obtaining inorganic drugs
Objective: To master the technology for receiving and manufacturing of inorganic drugs.
Learning objectives:
Apply some of the techniques for the synthesis in the laboratory.
Practice basis physical methods for processing drugs (filtration, crystallisation, distillation, redistillation, preparation of saturated solutions, etc.).
Разрешить написание латиницей
The main issue in this topic:
1. Common methods of work in chemical synthesis. 2. Admission calculate the theoretical yield of the drug, if possible, the expected yield on the conditions of synthesis of drugs. 3. Analysis methods for the synthesis of barium sulfate.
Methods of learning and teaching: analysis of theoretical issues and practical tasks on the synthesis of inorganic medicinal substances.
References:
Альбицкая В.Г., Гинзбург О.Ф., Коляскина З.Н., Купин Б.С., Павлова Л.А., Разумова Н.А., Ралль К.Б., Серкова В.И., Стадничук М.Д. Лабораторные работы по органической химии. Под ред. О.Ф. Гинзбурга, А.А. Петрова. М.: Высш. шк., 1967. – 295 с.
Майофис Л.С. Химия и технология химико-фармацевтических препаратов. 2-е изд., перераб. и доп. – М.: Медицина, 1964. – 625 с.
Control
1. What methods are used for separation and purification of substances in technological processes?
2. What physical methods are used for technological processes
3. Describe the basic operating time of obtaining barium sulfate for X-rays?
APPENDIX
lab work 1 - receiving barium sulfate for X-ray
Technique. The flask was poured 60 ml of water, to heat, dissolve 24.43 g of barium chloride with stirring with a glass rod. On cooling, the solution was adjusted to a density of 1.25, adding water or crystalline chlorideBarium (with areometer).
Separately, in a glass, a solution of sodium sulfate (in stoichiometric calculations). Dissolution are slight heating, prepare a saturated solution (1 g about 6 ml of water), add dilute sulfuric acid until acidic (pH 3). The solution is heated to a temperature of 60-80 0 and stirring, pouring in a slow stream of barium chloride solution (solutions should be pre-filtered). Settled and the resulting precipitate was washed with hot (decanting of the filter) to neutral to methyl orange. The wash water must not contain barium ions, chloride, sulfate.
Pure barium sulfate paste or suction filtered and dried at 100ć in the oven.
Lab.work 2 - Determination of the theoretical and practical output of barium sulfate for X-ray
The output of the drug should be about 90% of the theoretically calculated.
Quantity of finished product, g
η = ______________________________ х 100 %
Quantity of starting material, g
losses, g
έ (spending) = _______________________________ х 100 %
Quantity of starting material, g
Quantity of starting material, g
К expense = __________________________
Quantity of finished product, g

