
- •Материалы для студентов из учебно-методического комплекса по дисциплине
- •Специальность: лечебное дело – 040100
- •Смоленск 2008
- •Цели и задачи дисциплины
- •Требования к уровню освоения содержания дисциплины
- •Перечень дисциплин,
- •Тематическое содержание преподаваемого раздела хирургии
- •Раздел 1. Абдоминальная хирургическая патология
- •Тема 1. Абдоминальный болевой синдром
- •Тема 2. Острый аппендицит
- •Тема 3. Вентральные грыжи и их осложнения
- •Тема 4. Осложненный холецистит, вопросы хирургии желчных путей
- •Тема 5. Острый панкреатит
- •Тема 6. Хронический панкреатит, кисты поджелудочной железы
- •Тема 7. Рак и гормональноактивные опухоли поджелудочной железы
- •Тема 8. Механическая желтуха, холангит
- •Тема 9. Осложнения язв желудка и двенадцатиперстной кишки
- •Тема 10. Болезни оперированного желудка
- •Тема 11. Гастроэзофагеальная рефлюксная болезнь
- •Тема 12. Кишечная непроходимость
- •Тема 13. Хроническая дуоденальная непроходимость
- •Тема 14. Кишечные свищи
- •Тема 15. Перитонит
- •Тема 16. Объемные заболевания печени
- •Тема 17. Хирургия селезенки
- •Тема 18. Забрюшинные опухоли
- •Тема 19. Заболевания диафрагмы.
- •Тема 20. Современные технологии в диагностике и лечении хирургических заболеваний.
- •Тема 21. Осложненный рак желудка.
- •Раздел 2. Кровотечения
- •Тема 22. Гастродуоденальные кровотечения
- •Тема 23. Легочные кровотечения
- •Раздел 3. Хирургия повреждений мирного времени
- •Тема 24. Травма живота мирного времени, торакоабдоминальные ранения мирного времени
- •Тема 25. Хирургия повреждений груди
- •Раздел 4. Ургентная сосудистая хирургическая патология
- •Тема 26. Тэла у хирургических больных и её профилактика.
- •Тема 27. Острые артериальные тромбозы и эмболии.
- •Тема 28. Тромбоз и эмболия мезентериальных сосудов
- •Тема 29. Воспалительные заболевания вен нижних конечностей
- •Тема 30. Гангрена на фоне облитерирующих заболеваний артерий нижних конечностей
- •Тема 31. Портальная гипертензия
- •Тема 38. Предраковые заболевания толстой кишки
- •Тема 39. Рак толстой кишки
- •Раздел 6. Гнойно-воспалительные заболевания
- •Тема 40. Раны и раневая инфекция
- •Раздел 7. Костно-суставная хирургическая патология
- •Тема 51. Остеомиелит
- •Темы и план лекций для студентов
- •Осложненный аппендицит. Клинические «маски» острого аппендицита.
- •Заболевания, имитирующие «острый живот». Хирургические осложнения инфекционных и паразитарных заболеваний.
- •Ущемленные грыжи. Диагностика. Ошибки, опасности, особенности и осложнения в хирургическом лечении грыж.
- •Перитонит. Классификация, патогенез эндотоксикоза, клиника Лечение перитонита, перитонеальный лаваж, программированная релапаротомия, лапаростомия.
- •Осложненный холецистит.
- •Механическая желтуха.
- •Современная диагностика и дифференцированное лечение желудочно-кишечных кровотечений.
- •Повреждения груди мирного времени.
- •Повреждения живота мирного времени.
- •Осложненный рак желудка.
- •Осложненный рак прямой кишки.
- •Осложненный рак ободочной кишки.
- •Геморрой и его осложнения. Современные методы лечения.
- •Частные вопросы неотложной колопроктологии.
- •Легочные кровотечения.
- •Послеоперационные тромбоэмболические осложнения. Профилактика и лечение.
- •Ишемическая болезнь органов пищеварения. Острые нарушения мезентериального кровообращения.
- •Хирургический сепсис, классификация, клиника. Лечение сепсиса и хирургического инфекционно-воспалительного эндотоксикоза.
- •Эндовидеохирургические технологии (прошлое, настоящее, будущее).
- •Дифференцированные подходы к лечению хронического панкреатита.
- •Современные методы лечения кишечных свищей.
- •Болезни оперированного желудка. Показания к хирургическим методам лечения.
- •Гастроэзофагеальная рефлюксная болезнь, показания к хирургическому лечению.
- •Синдром диабетической стопы.
- •Зоб. Клиника, дифференциальная диагностика, хирургическое лечение.
- •Рак поджелудочной железы.
- •Опухоли печени.
- •Забрюшинные опухоли.
- •Хроническая дуоденальная непроходимость.
- •Заболевания диафрагмы.
- •Хирургия селезенки.
- •Иммунная система человека при гнойно-воспалительных и опухолевых онкологических заболеваниях органов брюшной полости.
- •Факультативные лекции
- •История кафедры и клиники госпитальной хирургии.
- •Достижения трансплантологии.
- •Портальная гипертензия.
- •Элективный курс практических занятий
- •Тема 1.Экстренная видеолапароскопия
- •Тема 2. Видеолапароскопия в плановой хирургии
- •Тема 3. Основные принципы и тактика озонотерапии в хирургии
- •Тема 4. Применение в хирургической практике гипохлорита натрия, получаемого электрохимическим методом
- •Учебно-методическое обеспечение дисциплины
- •Виды экзаменационных рентгенограмм
- •Задача 2.
- •Задача 3.
- •Задача 4.
- •Задача 5.
- •Задача 6.
- •Задача 7.
- •Задача 8.
- •Задача 9.
- •Задача 10.
- •Задача 11.
- •Задача 12.
- •Задача 13.
- •Задача 14.
- •Задача № 15
- •Задача 16.
- •Задача 17.
- •Задача 18.
- •Задача 19.
- •Задача 20.
- •Задача 21.
- •Задача 22.
- •Задача 23.
- •Задача 24.
- •Задача 25.
- •Задача 26.
- •Задача 27.
- •Задача 28.
- •Задача 29.
- •Задача 30. Больной, 50 лет, предъявляет жалобы на слабость, головокружение, окрашивание кала в черный цвет.
- •Задача 31.
- •Вопросы:
- •Ответы:
- •Задача 32.
- •Вопросы:
- •Задача 33.
- •Ответы:
- •Задача 34.
- •Задача 35.
- •Задача 36.
- •Задача 37.
- •Вопросы:
- •Задача 38.
- •Задача 39.
- •Задача 40.
- •Задача 41.
- •Задача 42.
- •Задача 43.
- •Задача 44.
- •Задача 45.
- •Задача 46.
- •Задача 47.
- •Задача 48.
- •Задача 49.
- •Задача 50.
- •Задача 51.
- •Задача 52.
- •Задача 53.
- •Задача 54.
- •Задача 55.
- •Задача 56.
- •Задача 57.
- •Задача 58.
- •Задача 59.
- •Задача 60.
- •Задача 61.
- •Задача 62.
- •Задача 63.
- •Задача 64.
- •Задача 65.
- •Задача 66.
- •Задача 67.
- •Задача 68.
- •Задача 69.
- •Задача 70.
- •Задача 71.
- •Задача 72.
- •Задача 73.
- •Задача 74.
- •Задача 75.
- •Задача 76.
- •Задача 77.
- •Острый аппендицит (варианты течения, осложнения)
- •2. Основные понятия
- •Классификация (по Колесову)
- •3. Вопросы к занятию.
- •4. Вопросы для самоконтроля.
- •5. Рекомендуемая литература.
- •Грыжи брюшной стенки, внутренние грыжи. Ущемленные грыжи (дифференциальный диагноз, клиника и особенности хирургической тактики)
- •Острый холецистит (клиника, осложнения, методы исследования желчевыводящих путей, оперативное и консервативное лечение, показания холедохотомии, методы дренирования холедоха)
- •1. Цель изучения.
- •2. Основные понятия
- •3. Вопросы к занятию.
- •1Особенности течения у беременных и пожилых людей
- •4. Вопросы для самоконтроля.
- •5. Литература
- •Острый панкреатит (классификация, стадии воспалительного процесса, консервативное лечение, показания и варианты хирургического пособия)
- •4. Основные понятия
- •5. Вопросы к занятию
- •6. Вопросы для самоконтроля
- •7. Основная и дополнительная литература
- •Хронический панкреатит, кисты и рак поджелудочной железы
- •4. Основные понятия:
- •5. Вопросы к занятию
- •6. Вопросы для самоконтроля
- •7. Основная и дополнительная литература
- •Механическая желтуха, холангит (классификация, дифференциальная диагностика, хирургическая тактика). Дренирующие и реконструктивные операции на желчевыводящих путях
- •4. Основные понятия
- •5. Вопросы к занятию
- •7. Основная и дополнительная литература
- •Диагностика и хирургическая тактика при осложнениях язв желудка и двенадцатиперстной кишки (прободение, пилородуоденальный стеноз, малигнизация, пенетрация)
- •Гастродуоденальные кровотечения (классификация, диагностика, хирургическая тактика)
- •Тема занятия, его цели и задачи.
- •2. Основные понятия:
- •3. Вопросы к занятию:
- •4.Вопросы для самоконтроля
- •Повреждения живота мирного времени. Торакоабдоминальные ранения (тупая травма, проникающие ранения, изолированные и сочетанные повреждения внутренних органов)
- •Хирургия повреждений груди мирного времени.
- •Острые артериальные тромбозы и эмболии. Флеботромбоз. Тэла у хирургических больных и её профилактика
- •Эндовидеохирургические технологии в диагностике и лечении ургентной абдоминальной патологии
- •2. Основные понятия
- •3. Вопросы к занятию.
- •4. Вопросы для самоконтроля.
- •2Кровоснабжение органов брюшной полости.
- •5. Литература
- •Вопросы для самоконтроля.
- •Дифференцированное лечение кишечной непроходимости. Методы кишечной декомпрессии, энтеральный лаваж
- •Перитонит (классификация, клиника, хирургическая тактика). Показания и методы перитонеального лаважа. Показания к программированной релапаротомии. Лапаростомия
- •Методические указания для студентов 6 курса
- •4. Основные понятия
- •5. Вопросы к занятию
- •6. Вопросы для самоконтроля
- •7. Основная и дополнительная литература
- •Раны и раневая инфекция. Современные подходы в лечении трофических язв, пролежней. Профилактика их развития
- •Назовите наиболее часто встречающихся возбудителей гнойной инфекции;
- •Хирургические заболевания и сахарный диабет. Синдром диабетической стопы
- •Золоев г.К. Облитерирующие заболевания артерий. – м., 2004. – 432с.
- •Хирургический сепсис. Синдром системного воспалительного ответа на инфекцию. Токсико-септический шок
- •Хроническая венозная недостаточность. Тромбофлебит (классификация, клиника, диагностика, лечение). Трофические язвы венозной этиологии
- •Гангрена конечностей на фоне облитерирующих заболеваний артерий нижних конечностей (клиника, диагностика, лечение)
- •Дифференциальная диагностика и лечение гнойно-воспалительных заболеваний кожи и подкожной клетчатки: фурункул и фурункулез, карбункул, абсцесс, флегмона, рожа, эризипелоид
- •Дифференциальная диагностика и лечение гнойно-воспалительных заболеваний: гидраденит, лимфангит и лимфаденит, мастит
- •Панариций. Остеомиелит
- •Флегмоны кисти и стопы. Флегмона пространства пирогова-парона
- •4. Основные понятия
- •5. Вопросы к занятию
- •Основная:
- •Артрозы, артриты, бурситы, гигромы. Остеохондропатии: болезнь Осгуд-Шлаттера, Шейермана-Мау, Пертеса, Кинбека, Келлера I и Келлера II (этиология, клиника, диагностика, лечение)
- •4. Основные понятия
- •5. Вопросы к занятию
- •Основная:
- •Тема: колоректальный рак Цели изучения
- •1. Предопухолевая патология толс-той кишки.
- •2. Клинические классификации для опухолей ободочной и прямой кишок.
- •3. Метастазирование колоректаль-ного рака.
- •4. Клинические проявления.
- •Диагностические исследования.
- •6.Скрининг колоректального рака.
- •8. Реабилитация.
- •9.Отдаленные результаты.
- •Вариант 2.
- •Вариант 3.
- •Вариант 4.
- •На тестовый контроль исходного уровня знаний
- •1 Вариант
- •На тестовый контроль исходного уровня знаний
- •2 Вариант
- •На тестовый контроль исходного уровня знаний
- •3 Вариант
- •На тестовый контроль исходного уровня знаний
- •4 Вариант
- •Тестовые задания для входного контроля (факультет иностранных студентов)
- •Программированный контроль к зачетному занятию по циклу
- •Программированный контроль к зачетному занятию по циклу
- •Программированный контроль к зачетному занятию по циклу
- •Программированный контроль к зачетному занятию по циклу
- •Программированный контроль к зачетному занятию по циклу
- •Программированный контроль к зачетному занятию по циклу
- •Итоговый тестовый контроль на кафедре госпитальной хирургии Вариант 1
- •Вариант 2
- •Вариант 3
- •5. Наличие яичка в грыжевом мешке характерно для грыжи:
- •Вариант 4
- •78. Для фазы дегидратации в течении раневого процесса характерно:
- •Тестовые задания для итогового контроля (факультет иностранных студентов)
- •Учебно-клинической истории болезни
- •1. Расспрос больного.
- •1. Осмотр.
- •2. Пальпация.
- •3. Перкуссия.
- •6. Определение характера пульса.
- •7. Измерение артериального давления.
- •8. Измерение венозного давления.
- •Дигитальный метод дополнительного исследования
- •Пальцевое исследование прямой кишки.
- •Инструментальные манипуляции
- •1. Исследование прямой кишки с помощью ректального зеркала.
- •4. Венесекция.
- •5. Пункция плевральной полости.
- •6. Пункция полости перикарда.
- •7. Пункция брюшной полости (лапароцентез).
- •8. Пункция коленного сустава.
- •9. Блокады анестетиками.
- •Словарь терминов и персоналий (глоссарий)
- •Русско-латинский словарь необходимых медицинских терминов
Артрозы, артриты, бурситы, гигромы. Остеохондропатии: болезнь Осгуд-Шлаттера, Шейермана-Мау, Пертеса, Кинбека, Келлера I и Келлера II (этиология, клиника, диагностика, лечение)
Составитель ассистент О.Г.Шахбазян
Методические указания утверждены на методическом совещании кафедры госпитальной хирургии (протокол № 2 от 6 октября 2008 г.)
Зав. кафедрой______________(проф. С.А.Касумьян)
2008 г.
1. Тема занятия: Артрозы, артриты, бурситы, гигромы (классификация, клиника, диагностика, лечение). Остеохондропатии: болезнь Осгут-Шлаттера, Шейермана-Мау, Пертеса, Кинбека, Келлера I и Келлера II (этиология, клиника, диагностика, лечение).
2.Цель занятия: Изучение методов диагностики артрозов, артритов, бурситов и гигром. Изучение методов диагностики остеохондропатий. Определение показаний к оперативному лечению.
3.Задачи: Определение тактики в отношении конкретного больного в зависимости от тяжести заболевания, изучение методов консервативного лечения, профилактики осложнений, определение показаний к оперативному лечению, знание основных методов операций.
4. Основные понятия
Артроз.
Артрит.
Бурсит.
Гигромы.
Болезнь Осгут-Шлаттера.
Болезнь Шейермана-Мау.
Болезнь Пертеса.
Болезнь Кинбека.
Болезнь Келлера I.
Болезнь Келлера II.
5. Вопросы к занятию
Этиология артрозов.
Этиология и классификация артритов и бурситов.
Клиника, диагностика артрозов, артритов, бурситов и гигром.
Этиология, патогенез, клиника, диагностика остеохондропатий.
Методы диагностики заболеваний опорно-двигательной системы.
Методы консервативного и оперативного лечения артрозов, артритов, бурситов и гигром.
Особенность хирургической обработки гнойного очага.
Методы лечения при остеохондропатиях.
Основные осложнения и их профилактика.
Рекомендуемая литература.
Основная:
Исаков Ю.Ф. Хирургические болезни детского возраста: Учебник для студентов медицинских вузов (в 2-х томах). – 2006.
Клиническая хирургия: в 3 т. Национальное руководство / Под ред. В.С.Савельева, А.И.Кириенко. – 2008. (+CD)
Николаев А.В. Топографическая анатомия и оперативная хирургия. Учебник для студентов медицинских вузов. – 2007. – 784с.
Травматология. Национальное руководство / Под ред. Г.П.Котельникова, С.П.Миронова. – 2008. – 808с. (+CD)
Дополнительная:
Амбулаторная хирургия. Справочник практического врача /Под ред. проф. В.В.Гриценко, проф. Ю.Д.Игнатова. – СПб.: Издательский Дом «Нева»; М.: «Олма-Пресс Звездный мир», 2002. – 448с.
Пауткин Ю.Ф. Поликлиническая хирургия. – М.: Высшая школа, 2005. – 287с.
Методическая разработка кафедры по теме практического занятия.
МЕТОДИЧЕСКИЕ УКАЗАНИЯ ДЛЯ СТУДЕНТОВ
По дисциплине хирургические болезни
Для специальности лечебное дело – 040100
Кафедра госпитальной хирургии
Курс 6 курс
Семестр XI-XII
Факультет иностранных студентов
ГОУ ВПО «Смоленская государственная медицинская академия
Федерального агентства по здравоохранению и социальному развитию»
МЕТОДИЧЕСКИЕ УКАЗАНИЯ ДЛЯ СТУДЕНТОВ
ПО ДИСЦИПЛИНЕ хирургические болезни
ACUTE APPENDICITIS
(variants of its clinical features, complications)
Составитель доц. А.Ю.Некрасов
Методические указания утверждены на методическом совещании кафедры госпитальной хирургии (протокол № 2 от 6 октября 2008 г.)
Зав. кафедрой______________(проф. С.А.Касумьян)
2008 г.
QUESTIONS FOR HOMEWORK:
1. Anatomy
2. Classification
3. Pathophy
4. Clinical symptoms.
5. Diagnostics.
6. Differential diagnosis.
7. Complications of appendicitis.
8. Appendicitis during pregnancy.
9. Chronic or recurrent appendicitis.
10. Treatment.
11. Postoperative complications.
ANATOMY
Although the base of the appendix is consistently found of the cecum, can be located in a variety of locations. The most common location of the appendix is retrocecal but within the peritoneal cavity, because the most inferior portion of the cecum is within the peritoneal cavity. This situation occurs approximately 65% of the time. It is pelvic in location in 30% and retroperitoneal in 2% of the population. The tip of the appendix can also be found in a preileal or postileal location. The varying location of the appendix explains the myriad of symptoms that can be found in patients with appendicitis.
The appendicle artery, a branch of the ileocolic artery, supplies the appendix. Histological examination of the appendix shows a number of lymphoid follicles in the submucosa. The lumen of the appendix is often obliterated in elderly persons. The appendix in the adult can vary widely in length from 2 to 11 cm but averages about 9 cm in length.
CLASSIFICATION OF ACUTE APPENDICITIS (B.V. PETROVSKIY, 1980)
Appendicolitis (functional form).
Simple or catarrhal appendicitis.
Destructive appendicitis: phlegmone; gangrenous appendix, perforated and nonperforated.
Complication of appendicitis: periappendiceal infiltration; periappendiceal abscess; peritonitis; phlegmone of the mesenteric; sepsis; other complications.
PATHOPHYSIOLOGY
Appendicitis is caused by obstruction of the appendiceal lumen from a variety of causes. Independent of the etiology, obstruction is believed to cause an increase in pressure within the lumen. Such an increase is related to continuous secretion of fluids and mucus from the mucosa and the stagnation of this material. At the same time, intestinal bacteria within the appendix multiply, leading to the recruitment of white blood cells, pus formation and subsequent increase of intraluminal pressure.
If appendiceal obstruction persists, intraluminal pressure rises ultimately above that of the appendiceal veins, leading to venous outflow obstruction. As a consequence, appendiceal wall ischemia begins. It may resulting in a loss of epithelial integrity and allow bacterial invasion of the appendiceal wall.
Within a few hours, this localized condition may worsen because of thrombosis of the appendicular artery and veins. The mair veason of it’s thrombosis of veins and arteries that supply the appendix. This phenomenon leads to perforation and gangrene of the appendix.
CLINICAL SYMPTOMS
The typical history is one of an onset of generalized abdominal pain followed by anorexia and nausea. The pain then becomes most prominent in the epigastrium and gradually moves toward the umbilicus, finally localizing in the right lower quadrant (Cocher,s symptom).
Examination of the abdomen usually shows:
Diminished bowel sounds, with direct tenderness and muscle spasm in the right lower quadrant. As the process continues the amount of spasm increases, with the appearance of rebound tenderness.
The temperature is usually mildly elevated (approximately 38°C) and usually rises to higher levels in the event of perforation, although this is very variable.
Tenderness on palpation in the right lower quadrant is the most important sign in these patients.
Additional signs such as increasing pain with cough (ie, Dunphy sign)
Rebound tenderness related to peritoneal irritation elicited by deep palpation with quick release (ie, Blumberg sign), and guarding may or may not be present. Right lower quadrant pain might be increased when patient turns on the right side.
Rovsing sign, which is elicited when pressure applied in the left lower quadrant reflects pain in the right lower quadrant, often is present but not specific.
The psoas sign (Bartomye-Michelson sign) may be positive and is elicited by extension of the right thigh with the patient lying on the left side. As the examiner extends the right thigh with stretching of the muscle, pain suggests the presence of an inflamed appendix overlying the psoas muscle.
The obturator sign (Coup sing) can be elicited with the patient in the supine position with passive rotation of the flexed right hip. Pain with this maneuver indicates a positive sign.
Obrazcov sign –increasing pain elicited when the pressure applied in the right lower quadrant during the lifting and straightening of the right foot.
Rectal examination should be performed in any patient with an unclear clinical picture, and a pelvic examination should be performed in all women with abdominal pain.
If the appendix ruptures, abdominal pain becomes intense and more diffuse, the muscular spasm increases, and there is a simultaneous increase in the heart rate, with a rise in temperature to 39° to 40°C. Rarely, there may be a slight diminishing of pain with rupture, presumably due to the decreased distention of the appendix, but a true pain-free interval would be quite uncommon.
FURTHER WORKUP
Laboratory. The majority of patients with acute abdominal pain have a complete blood count as a component of the evaluation. The leukocyte count is usually elevated to the range of 12,000 to 18,000. In addition, an increase in the percentage of younger neutrophil forms (the "left shift") with a normal total white blood cell count supports the clinical diagnosis of appendicitis. A completely normal leukocyte count differential is uncommon in patients with appendicitis. Other laboratory indices of inflammation have been studied as adjuncts to diagnosis of appendicitis.
A urinalysis is often obtained in the evaluation of patients with abdominal pain to determine whether genitourinary tract inflammation is present. The urinalysis may show a mild pyuria with appendicitis owing to the proximity of the ureter to the inflamed appendix. The increased specific gravity of the urine adds to the clinical diagnosis of hypovolemia. Proteinuria can also be an adjunct to the diagnosis of spontaneous bacterial peritonitis as a complication of nephrotic syndrome in children evaluated for acute abdominal pain.
Ultrasound. Ultrasound (US) is noninvasive and rapidly available and avoids radiation exposure. However, the sonogram for appendicitis is a highly operator-dependent study. A healthy appendix usually cannot be viewed with US. When appendicitis occurs, the US typically demonstrates a noncompressible tubular structure of 7-9 mm in diameter. Interruption of the continuity of the echogenic submucosa, appendicolith and periappendiceal fluid or mass might be observed.
Abdominal radiographs. This study may be useful in patients with atypical presenting symptoms and physical signs. Abdominal radiographs may demonstrate a fecalith, localized ileus, or loss of the peritoneal fat stripe.
Computed tomography. Typically, CT has been reserved for patients with an equivocal history and physical and laboratory findings. CT is useful in patients with an observed inflammatory abdominal process, and the presentation is atypical for appendicitis.
Nuclear medicine. There is a renewed interest in the use of nuclear medicine studies to evaluate patients with suspected appendicitis. The true potential usefulness of these studies occurs in patients with persistent symptoms and negative US and CT studies.
DIFFERENTIAL DIAGNOSIS
There are some common diagnoses to consider in the differential of appendicitis including:
Diseases of abdominal cavity organs: acute cholecystitis, acute pancreatitis, gastric and duodenum ulcers, intussusception, Crohn's disease, Meckel's diverticulitis and others;
Urologic diseases: renal colic, nephrolithiasis, paranephritis, acute pyelonephritis, and urinary tract infection;
Gynecological diseases: ovarian cyst or torsion, ectopic pregnancy, ovarian tumor, endometritis, acute adnexitis;
Infectious diseases: acute gastroenteritis, perityphlitis, enterokolitis digestive disorder;
Thoracic diseases: pneumonia, pleurisy, myocardial infarction, coronary artery disease;
The large majority of patients can be diagnosed and treated on the basis of a good history, physical examination, and judicious use of laboratory tests.
COMPLICATIONS OF APPENDICITIS
Periappendiceal infiltration – the inflammatory conglomeration, which consist of appendix and prepossessing near organs and tissues. It may be friable and compact.
Periappendiceal abscess – it is formed after the suppuration of infiltration. The leukocyte count and ESR usually elevated, the "left shift" is found. The temperature is usually raised to higher levels.
There also has been a bias toward early removal of the perforated appendix/appendiceal abscess to "control intra-abdominal sepsis." The preferred approach to the management of the appendiceal mass is percutaneous drainage, which is performed under image guidance (ultrasound or CT) and intravenous antibiotics directed against aerobic gram-negative and anaerobic organisms, followed by interval appaendectomy. Numerous studies have documented the safety and efficacy of this approach.
In late, complicated appendicitis, appendectomy can be a hazardous procedure. Surgery at this stage can serve to disseminate a localized inflammatory process; to injure surrounding inflamed or edematous bowel, resulting in fistulas; or to require more extensive procedures, such as cecectomy or right hemicolectomy. Historically, this has been fueled by equivocal imaging studies that could not reliably corroborate the physical findings and an inability to reliably drain an abscess percutaneously.
Perforated appendicitis. Patients with perforated appendicitis will often have a longer duration of symptoms, high fever, and a higher white blood cells count. Most of these patients are volume depleted and require several hours or more of fluid resuscitation before operative intervention. It is important to ensure that the patient has been adequately resuscitated before undertaking an operation. Patients with perforated disease have established peritonitis and should receive appropriate broad-spectrum intravenous antibiotic therapy, which should start as soon as the diagnosis is established. The duration of therapy is controversial. Some authors recommend an empiric time of treatment such as 7 or 10 days. Others suggest treatment until the patient is afebrile with a normal white blood cell count.
As with acute appendicitis, there are two possible approaches: an open laparotomy or laparoscopy. There is some controversy about the use of laparoscopy in patients with advanced disease because the incidence of postoperative intra-abdominal abscess formation in some series has been markedly higher with laparoscopy than with an open approach.
APPENDICITIS DURING PREGNANCY
Appendicitis and cholecystitis are the most frequent causes of abdominal pain during pregnancy. Abdominal tenderness is the most important finding in appendicitis, but the location of point tenderness varies during gestation. After the fifth month of gestation, the appendiceal position is shifted superiorly above the iliac crest, and the appendix tip is rotated medially by the gravid uterus.
The whole blood cell count may not be helpful because it is frequently elevated during pregnancy. Common symptoms such as nausea, vomiting, anorexia are also common during pregnancy and thus of limited diagnostic value. Ultrasound may be of help if a thickened or dilated appendix is identified.
Suspicion of appendicitis should lead to early surgical intervention in all trimesters. Negative laparotomy results in minimal fetal loss, whereas a delay in diagnosis and perforation may result in a high incidence of fetal death and a relatively high incidence of maternal death. A laparoscopic approach has been used and does not appear to increase maternal or fetal morbidity or mortality rates.
CHRONIC OR RECURRENT APPENDICITIS
The occurrence of chronic or recurrent appendicitis is controversial, and although rare, its existence may be plausible. Intermittent bouts of obstruction of the appendiceal lumen with spontaneous remission may be the cause. Mild local inflammation after a resolving attack of acute appendicitis may result in chronic right lower quadrant discomfort.
The appearance of the appendix on CT in patients with recurrent or chronic appendicitis reportedly demonstrates findings similar to acute appendicitis. Patients undergoing appendectomy for chronic lower abdominal pain frequently demonstrate abnormal histology of the appendix and are relieved of their symptoms.
TREATMENT
The treatment of appendicitis depends on the stage of the disease. In general, patients should receive fluid resuscitation before surgery, but this may require only 1 or 2 hours in patients with nonperforated disease. There is a general consensus that prophylactic antibiotics should be administered before the start of the operation, a single dose of cefoxitin or cefotaxime is administered.
There are two approaches to removal of the nonperforated appendix: through an open incision, usually a transverse right lower quadrant skin incision (Davis-Rockey) or an oblique version (McArthur-McBurney) with separation of the muscles in the direction of their fibers, or a paramedian incision, but this is not routinely done. The incision is centered on the medioclavicular line (Fig.1). Once the peritoneum is entered, the appendix is delivered into the field. This can usually be accomplished with careful digital manipulation of the appendix and cecum. In difficult cases, extending the incision 1 to 2 cm can greatly simplify the procedure. Once the appendix is delivered into the wound, the mesoappendix is sacrificed between clamps and ties (Fig.2). There are several ways to handle the actual removal of the appendix. Some surgeons simply suture ligate the base of the appendix and excise it. Others place a pursestring or Z – stitch in the cecum, excise the appendix, and invert the stump into the cecum (Fig.3). Once the appendix is removed, the cecum is returned to the abdomen, and the peritoneum is closed.
The appendix can also be removed laparoscopically. Although there is not universal agreement, the body of information suggests that in the adult, although operative costs are higher due to a longer procedure and more equipment needed with laparoscopy, the overall costs are lower because the pain is less and patients can return to work sooner.
In a laparoscopic procedure appendix is grasped by atraumatic grasper and retracted upward to expose the mesoappendix. The mesoappendix is divided using a dissector then ligated with a linear Endostapler, Endoclip, or suture ligature. The mesoappendix is transected using a scissor or electrocautery. To avoid perforation of the appendix and iatrogenic peritonitis, the tip of the appendix should not be grasped. The appendix may now be transected with a linear Endostapler, or, alternately, the base of the appendix may be suture ligated in a similar manner to that in an open procedure. The appendiceal stump is not buried, and the the appendix is removed through one of the port sites. Peritoneal irrigation is performed with antibiotic or saline solution. The fascial layers are closed with absorbable suture, while the cutaneous incisions are closed with interrupted subcuticular sutures or sterile adhesive strips.
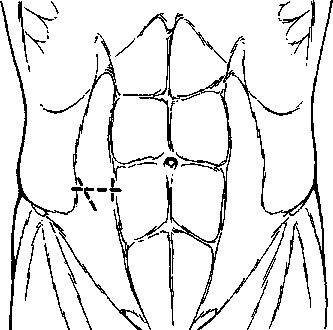
Figure 1. Location for the common incisions used for an appendectomy.
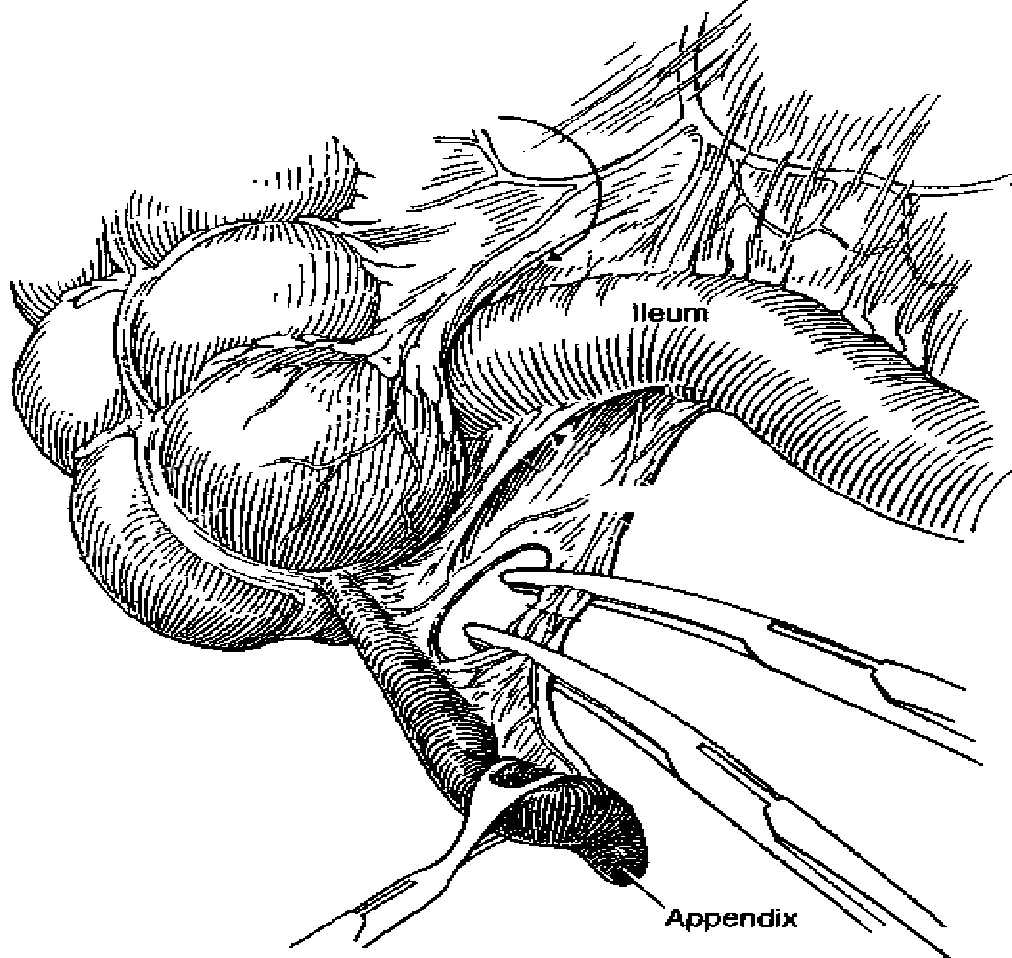
Figure 2.Division of the mesoappendix during an open appendectomy
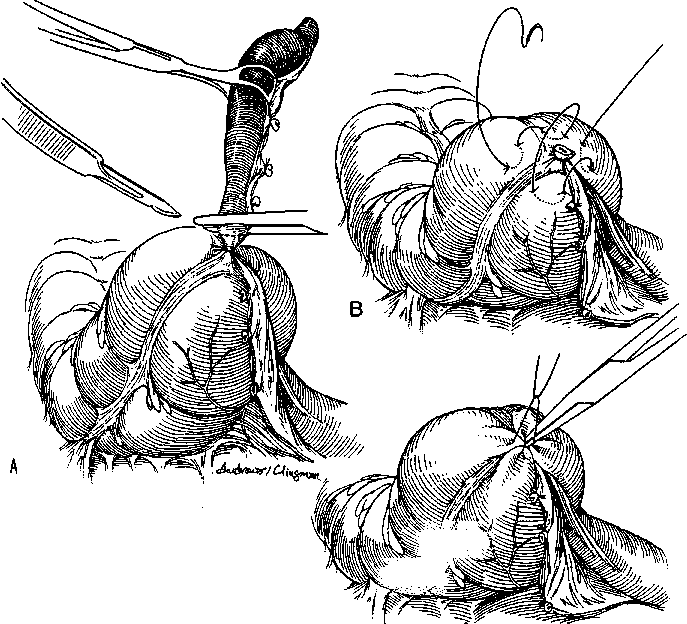
Figure 3. Steps in an open appendectomy. A. The appendix is divided after ligation. B. A Z or pursestring stitch is placed in the cecum. C. Inversion of the appendiceal stamp
POSTOPERATIVE COMPLICATIONS
Infection. Infection remains the most common complication after the operative treatment of appendicitis. Although infection can occur in a number of locations, surgical site infection predominates. The two sites at which infections can occur are the subcutaneous wound and within the abdominal cavity.
The incidence of wound infection and intra-abdominal sepsis in patients with complicated appendicitis is higher than that in patients with nonperforated appendicitis. There have been several reports of a much higher incidence of abscess formation in patients with complicated appendicitis who have undergone laparoscopic appendectomy. The mechanism is unclear at this time.
Bowel obstruction. Intestinal obstruction can occur after laparotomy for appendicitis. The true long-term incidence is unknown, but it is likely similar to the risk of patients undergoing laparotomy for other reasons.
Infertility. The risk of tubal infertility in female patients after appendicitis is unclear. In one large study, there was no increased risk of infertility in patients with nonperforated appendicitis but a several-fold increase in infertility in patients with perforated appendicitis. However, another study showed no difference in either group. Regardless, the risk appears to be sufficiently low that patients do not require routine evaluations unless there is a proven problem with fertility.
Miscellaneous. As with any operation, a number of other problems may occur. Complications which can occur in patients with appendicitis:
urinary tract infections’
pneumonia
a fecae fistula
TESTS
1 . The Earliest symptoms of acute appendicitis is
a) Pain
b) Fever
c) Vomiting
d) Rise of pulse rate
2. The Commonest position of the appendix -
a) Retrocaecal
b) Preileal
c) Postileal
d) Pelvic
e) Subcaecal
3. Which of the following present as acute abdomen
a) Acute intermittent porphyria
b) Tabes
c) Pneumonitis of lower lobe
d)All
4. Oschner sherren regime is used in the management of-
a) Appendicular abscess
b) Chronic appendicitis
c) Appendicular mass
d) Acute appendicitis
5. Acute appendicitis is due to -
a) Faecoliths
b) Worms of ileo-caecal region
c) Streptococcal infections
d) Abuse of puragatives
e) None of the above
6. All of the following are early complications after appendectomy for acute appendicitis except-
a) ileus
b) Sterility
c) Intestinal obstruction
d) Pulmonary complications
7. Acute appendicitis is best diagnosed by -
a) History
b) Physical examination
c) X-ray abdomen
8. During appendectomy if it is noticed that the base of appendix is inflamed than further line of treatment is-
a) No appendectomy
b) No burying of stump
c) Hemicolectomy
d) Caecal resection
9. A 25 year old man presents with 3 days history of pain in the right lower abdomen and vomiting, patient's general condition is satisfactory and clinical examination reveals a tender lump the right iliac fossa. The most appropriate management in this case would be-
a) Immediate appendectomy
b) Exploratory laparotomy
c) Oschner Sherren regimen
d) External drainage
10. All of the following signs are not seen in acute appendicitis except -
a) Rovsing's
b) Murphy's sign
c) Boa's sign
d) Mack wen's sign
ГОУ ВПО «Смоленская государственная медицинская академия
Федерального агентства по здравоохранению и социальному развитию»
МЕТОДИЧЕСКИЕ УКАЗАНИЯ ДЛЯ СТУДЕНТОВ
ПО ДИСЦИПЛИНЕ хирургические болезни
EXTERNAL AND INTERNAL HERNIAS. STRANGULATED HERNIAS (differential diagnosis, clinical features, surgical tactics)
Составитель асс. А.Л.Буянов
Методические указания утверждены на методическом совещании кафедры госпитальной хирургии (протокол № 2 от 6 октября 2008 г.)
Зав. кафедрой______________(проф. С.А.Касумьян)
2008 г.
HERNIA
A hernia is the protrusion of a viscus or part of a viscus through an abnormal opening in the walls of its containing cavity. The external abdominal hernia is the commonest form of spontaneous hernia, and mostly of the inguinal, femoral and umbilical varieties, respectively 73, 17 and 8.5 per cent (Fig. 1). This leaves 1.5 per cent for the rarer forms. Incisional hernia is another increasingly common variety of external acquired hernia.
.
Fig. 1. External hernias.
Red = common; white = not unusual; black = rare
GENERAL FEATURES COMMON TO ALL HERNIAS
Aetiology. A powerful muscular effort or strain occasioned by lifting a heavy weight, or indeed any condition which raises intra-abdominal pressure, is liable to be followed by a hernia. Whooping cough is a predisposing cause in childhood, while a chronic cough, straining on micturition or on defaecation may precipitate a hernia in an adult. It should be remembered that the appearance of a hernia in an adult can be a sign of intra-abdominal malignancy. Premature infants have a high incidence of hernia.
Stretching of the abdominal musculature because of an increase in contents, as in obesity and in pregnancy, can be another factor. Fat acts as a kind of 'pile-driver' for it separates muscle bundles and layers, weakens aponeuroses, and favours the appearance of paraumbilical, direct inguinal, and hiatus hernias.
Inguinal hernia is commoner (x20) in men, and is probably present in 5-10 per cent of the normal male population. African men have a much higher incidence of inguinal hernia than Europeans.
Raised intraabdominal pressure (e.g. from ascites) can promote the development of all types of hernia.
Composition of a hernia. As a rule, a hernia consists of three parts - the sac, the coverings of the sac, and the contents of the sac.
The sac is a diverticulum of peritoneum consisting of mouth, neck, body and fundus. The neck is usually well defined, but in some direct inguinal hernias and in many incisional hernias there is no actual neck. The diameter of the neck is important, because strangulation of bowel is a likely complication where the neck is narrow, as in femoral and umbilical hernia.
The body of the sac varies greatly in size and is not necessarily occupied. In cases occurring in infancy and childhood the sac is gossamer thin. In long-standing cases, especially after years of pressure by a truss, the wall of the sac is comparatively thick.
Coverings are derived from the layers of the abdominal wall through which the sac passes. In long-standing cases they become atrophied from stretching and so amalgamated that they are indistinguishable one from another.
Contents. These can be almost any abdominal viscus, except the liver, but the commonest are:
• fluid, the most common content, derived from peritoneal exudate. It can also appear as a part of ascites, or as a residuum thereof. Blood-stained fluid accompanies strangulation;
• omentum = omentocele (syn. epiplocele);
• intestine = enterocele. Usually small intestine, but, in some instances, large intestine or the vermiform appendix;
• a portion of the circumference of the intestine = Richter's hernia;
• a portion of the bladder, or a diverticulum of the bladder, is sometimes present in addition to other contents in a direct inguinal, a sliding inguinal, and in a femoral hernia;
• ovary with or without the corresponding Fallopian tube;
• Meckel's diverticulum = Littre's hernia.
Classification
Irrespective of site, a hernia can be:
1. reducible
2. irreducible (Complication of 1)
3. obstructed
4. strangulated} (Complications of 2)
5. inflamed.
Reducible hernia. The hernia either reduces itself when the patient lies down, or can be reduced by the patient or by the surgeon. Note that intestine gurgles on reduction, and the first portion is more difficult to reduce than the last. Omentum is doughy, and the last portion is more difficult to reduce than the first. A reducible hernia imparts an expansile impulse on coughing.
Irreducible hernia. Here the contents cannot be returned to the abdomen and there is no evidence of other complications. It is brought about by adhesions between the sac and its contents or from overcrowding within the sac. Irreducibility without other symptoms is almost diagnostic of an omentocele especially in femoral and umbilical hernia. Note: Any degree of irreducibility predisposes to strangulation.
Obstructed hernia. (Syn. incarcerated hernia. The term 'incarceration' is often used loosely as an alternative to irreducibility, obstruction or strangulation. Because of its lack of precision, the term should not be used to describe a complicated hernia.) This is an irreducible hernia containing intestine which is obstructed from without or from within; but there is no interference to the blood supply to the bowel. The symptoms are less severe and the onset more gradual than is the case in strangulation, but more often than not the obstruction culminates in strangulation. Often no clear distinction can be made between obstruction and strangulation in hernias, so the safe course is to assume that strangulation is imminent and to treat accordingly.
Strangulated hernia. A hernia becomes strangulated when the blood supply of its contents is seriously impaired, rendering gangrene imminent. Gangrene may occur as early as 5 or 6 hours after the onset of the first symptoms of strangulation. Although inguinal hernia is four times more common than femoral hernia, a femoral hernia is more likely to strangulate because of the narrowness of the neck of the sac and its rigid walls.
Pathology. The intestine is obstructed (except in a Richter's hernia, see below) and in addition its blood supply is constricted. At first only the venous return is impeded. The wall of the intestine becomes congested and bright red, and serous fluid is poured out into the sac. As the congestion increases, the intestine becomes purple in colour. As a result of increased intestinal pressure the strangulated loop becomes distended, often to twice its normal diameter. As venous stasis increases, the arterial supply becomes more and more impaired. Blood is extravasated under the serosa (an ecchymosis) and is effused into the lumen. The fluid in the sac becomes bloodstained. The shining serosa becomes dull and covered by a fibrinous, sticky exudate. By this time the walls of the intestine have lost their tone; they are flabby, and are very friable. The lowered vitality of the intestine favours migration of bacteria through the intestinal wall, and the fluid in the sac teems with bacteria. Gangrene appears first at the rings of constriction (Fig. 2), which become deeply furrowed and grey in colour, and then it appears in the antimesenteric border and spreads upwards, the colour varying from black to green according to the decomposition of blood in the subserosa. The mesentery involved by strangulation also becomes gangrenous. If the strangulation is unrelieved, perforation of the wall of the intestine occurs, either on the convexity of the loop or at the seat of constriction. Peritonitis spreads from the sac to the peritoneal cavity.
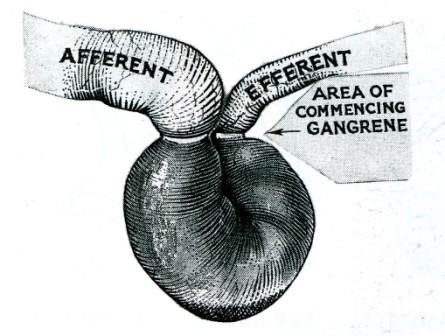
Fig. 2. Gangrene commences at the areas of constriction and then at the antimesenteric border.
Clinical features. Sudden pain, at first situated over the hernia, is followed by generalised abdominal pain, paroxysmal in character and often located mainly at the umbilicus. Vomiting is forcible and usually oft-repeated. The patient may say that the hernia has recently become larger. On examination, the hernia is tense, extremely tender, irreducible and there is no expansile impulse on coughing.
Unless the strangulation is relieved by operation, the paroxysms of pain continue until peristaltic contractions cease with the onset of gangrene when paralytic ileus (often the result of peritonitis) and toxic shock develop. Spontaneous cessation of pain is therefore of grave significance.
Richter's hernia is a hernia in which the sac contains only a portion of the circumference of the intestine (usually small intestine). It usually complicates femoral and, rarely, obturator hernias.
Strangulated Richter's hernia (Fig. 3) is particularly dangerous as operation is frequently delayed because the strangulated knuckle of bowel is small and there is no obstruction to the bowel lumen. A femoral site is common for this hernia and in a fat woman the local signs of strangulation are often not obvious. The patient may not vomit, or vomits only once or twice. Intestinal colic occurs, but the bowels are often opened normally or there may be diarrhoea; absolute constipation is delayed until paralytic ileus supervenes. For these reasons gangrene of the knuckle of bowel and peritonitis often have occurred before operation is undertaken.
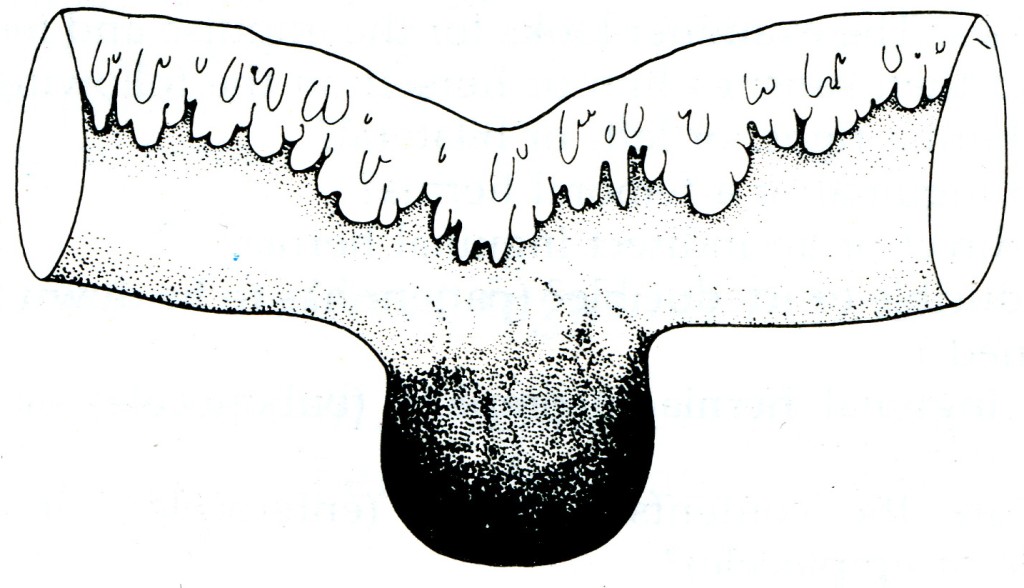
Fig. 3. Gangrenous Richter's hernia from a case of strangulated femoral hernia.
Strangulated omentocele. The initial symptoms are in general similar to those of strangulated bowel. Vomiting and constipation may be absent. Unlike intestine, omentum can exist on a very meagre blood supply. The onset of gangrene is therefore correspondingly delayed, and it occurs first in the centre of the fatty mass. Unrelieved, a bacterial invasion of the dying contents of the sac will almost certainly occur. Infection is limited to the sac for days, and sometimes for weeks. In an inguinal hernia, infection usually terminates as a scrotal abscess, but extension of peritonitis from the sac to the general peritoneal cavity is always a possibility.
Inflamed hernia. Inflammation can occur from irritation or sepsis of the contents within the sac, e.g. acute appendicitis or salpingitis, also from external causes, e.g. from a sore caused by an ill-fitting truss. The hernia is tender but not tense, and the overlying skin becomes red and oedematous. Operation is necessary to deal with the cause.
INGUINAL HERNIA
Surgical anatomy
The superficial inguinal ring is a triangular aperture in the aponeurosis of the external oblique, and lies 1.25 cm above the pubic tubercle. The ring is bounded by a superomedial and an inferolateral crus joined by criss-cross intercrural fibres. Normally the ring will not admit the tip of the little finger (Fig. 4).
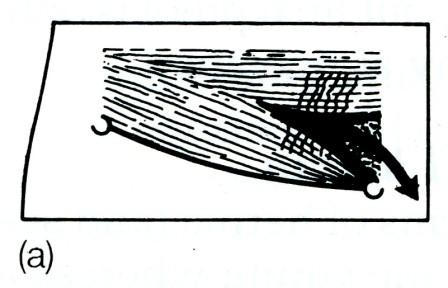
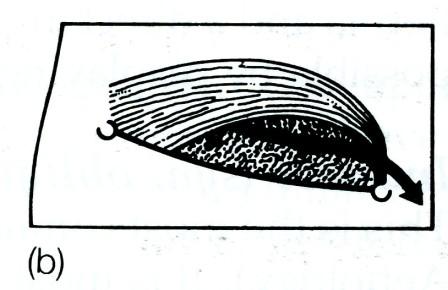
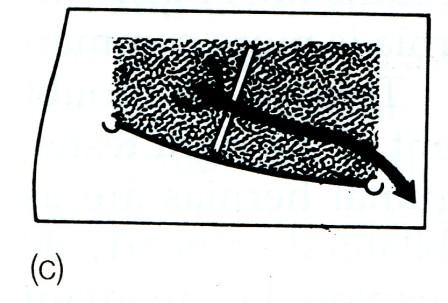
Fig. 4. The boundaries of the right inguinal canal. The inguinal ligament passes between the anterior superior iliac spine laterally, and the pubic tubercle medially, (a) The superficial layer, the external oblique aponeurosis, the crura of the external ring and the intercrural fibres; (b) the conjoined muscle (internal oblique and transversus) arching over the cord. Laterally the conjoined muscle lies superficial to the cord and the internal ring, then above the cord and medially, as the conjoined tendon, behind the cord; (c) the deepest layer which is the transversalis fascia (the fascial envelope of the abdomen). The inferior epigastric artery is shown lying medial to the internal ring.
The deep inguinal ring is a U-shaped opening in the transversalis fascia 1.25 cm above the midpoint of the inguinal ligament (Pouparfs ligament). The transversalis fascia is the fascial envelope of the abdomen, and the competency of the deep inguinal ring depends upon the integrity of this fascia.
The inguinal canal. In infants the superficial and deep inguinal rings are almost superimposed, and the obliquity of the canal is slight. In adults the inguinal canal, which is about 3.75 cm long, is directed downwards and medially from the deep to the superficial inguinal ring. In the male the inguinal canal transmits the spermatic cord, the ilioinguinal nerve, and the genital branch of the genito-femoral nerve. In the female the round ligament replaces the spermatic cord.
Boundaries of the inguinal canal. The best way to understand these is to study Fig. 4 (viewing the canal from the superficial to the deep layers as is seen at operation).
Thus, the boundaries of the inguinal canal are as follows:
Anteriorly. (Fig. 4a and b). External oblique aponeurosis. The conjoined muscle (mainly internal oblique) laterally.
Posteriorly. Inferior epigastric artery; fascia transversalis; conjoined tendon (internal oblique and transversus) medially (Fig. 4c and b).
Superiorly. Conjoined muscles (internal oblique and transversus) (Fig. 4b).
Inferiorly. Inguinal ligament (Fig. 4a, b and c).
Distinction between indirect and direct inguinal hernias, and a femoral hernia. An indirect inguinal hernia travels down the canal on the outer (lateral and anterior) side of the spermatic cord. A direct hernia comes out directly forwards through the posterior wall of the inguinal canal. While the neck of an indirect hernia is lateral to the inferior epigastric vessels, the direct hernia usually emerges medial to this except in the saddle-bag or pantaloon type, which has both a lateral and a medial component. An inguinal hernia can be differentiated from a femoral hernia by ascertaining the relation of the neck of the sac to the medial end of the inguinal ligament and the pubic tubercle, i.e. in the case of an inguinal hernia the neck is above and medial, while that of a femoral hernia is below and lateral (Fig.14). Digital control of the internal ring will help in distinguishing between an indirect inguinal hernia and a direct inguinal hernia, but final proof is only possible by displaying the anatomy at operation.
Indirect (syn. oblique) inguinal hernia
This is the most common of all forms of hernia (and see Aetiology). It is most common in the young whereas a direct hernia is most common in middle life or after. In the first decade of life inguinal hernia is more common on the right side in the male. This is no doubt associated with the deferred descent of the right testis. After the second decade, left inguinal hernias are as frequent as right. The hernia is bilateral in nearly 30 per cent of cases. If both sides are explored in an infant presenting with one hernia, the incidence of a patent processus vaginalis on the other side is 60 per cent. The condition usually occurs in a preformed sac (remains of processus vaginalis).
Three types of oblique inguinal hernia occur (Fig. 5).
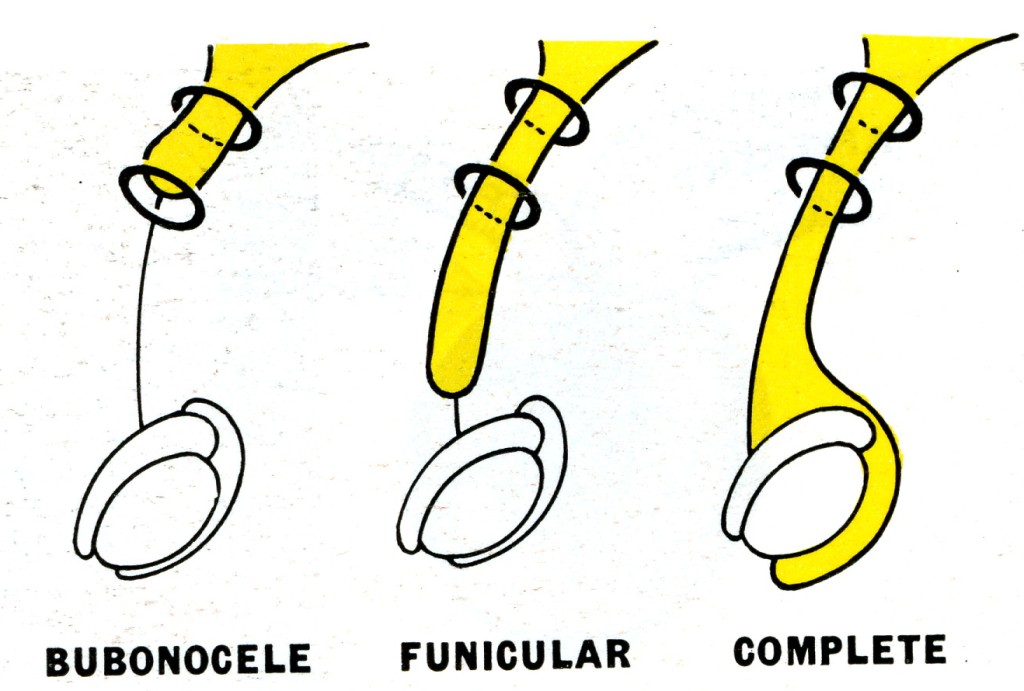
Fig. 5. Types of oblique inguinal hernia. Bubon (Greek) = groin; funiculus (Latin) = a small cord.
Bubonocele. When the hernia is limited to the inguinal canal.
Funicular. The processus vaginalis is closed just above the epididymis. The contents of the sac can be felt separately from the testis, which lies below the hernia.
Complete (syn. scrotal). A complete inguinal hernia is rarely present at birth but is commonly encountered in infancy. It also occurs in adolescence or adult life. The testis appears to lie within the lower part of the hernia.
Clinical features. Occurring at any age, males are 20 times more commonly affected than females. The patient complains of pain in the groin or pain referred to the testicle when he is performing heavy work, or taking strenuous exercise. When he is asked to cough a transient bulging may be seen and felt, together with an expansile impulse (see below). When the sac is still limited to the inguinal canal, the bulge may be better seen by observing the inguinal region from the side or even looking down the abdominal wall while standing slightly behind the respective shoulder of the patient.
As an oblique inguinal hernia increases in size it becomes more obvious when the patient coughs, and persists until reduced (Fig. 6). As time goes on the hernia comes down as soon as the patient assumes the upright position. In large hernias there is a sensation of weight, and dragging on the mesentery may produce epigastric pain. If the contents of the sac are reducible, the inguinal canal will be found to be commodious.
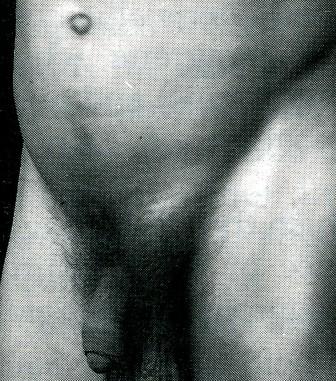
Fig. 6. Oblique left inguinal hernia which became apparent when the patient coughed, and persisted until it was reduced when he lay down.
In infants the swelling appears when the child cries. It can be translucent in infancy and early childhood, but never in an adult. In young girls an ovary may prolapse into the sac as the pelvis has not fully enlarged to accommodate the organs.
Notes on the clinical examination. The clinician is seated in front of the patient who stands with his legs apart. He is instructed to look at the ceiling and to cough at the ceiling. If the hernia will come down, it usually does. The examiner looks for the impulse and feels for the impulse and then satisfies him or herself on the following points:
• Is the hernia right, or left, or bilateral?
• Is it an inguinal or a femoral hernia?
• Is it a direct or an indirect inguinal hernia?
• Is it reducible or irreducible? (patient has to lie down for this to be ascertained.)
• Is the inguinal hernia incomplete (bubonocele) or complete (scrotal)?
• What are the contents - bowel (enterocele), or omentum (omentocele or epiplocele)?
Differential diagnosis in the male (Fig. 7):
• a vaginal hydrocele
• an encysted hydrocele of the cord;
• spermatocele;
• a femoral hernia;
• an incompletely descended testis in the inguinal canal. An inguinal hernia is often associated with this condition;
• a lipoma of the cord. This is often a difficult, but unimportant, diagnosis. It is usually not settled until the parts are displayed by operation.
Note: examination using finger and thumb across the neck of the scrotum will help to distinguish between a swelling of inguinal origin and one which is entirely intrascrotal.
Differential diagnosis in the female:
• a hydrocele of the canal of Nuck is the commonest differential diagnostic problem;
• a femoral hernia.
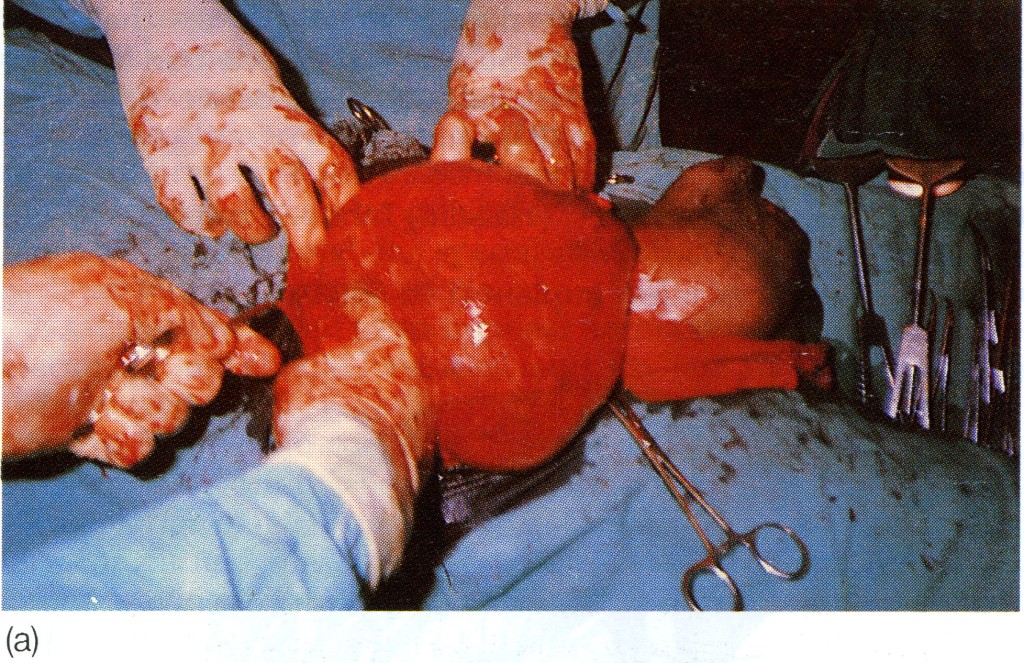
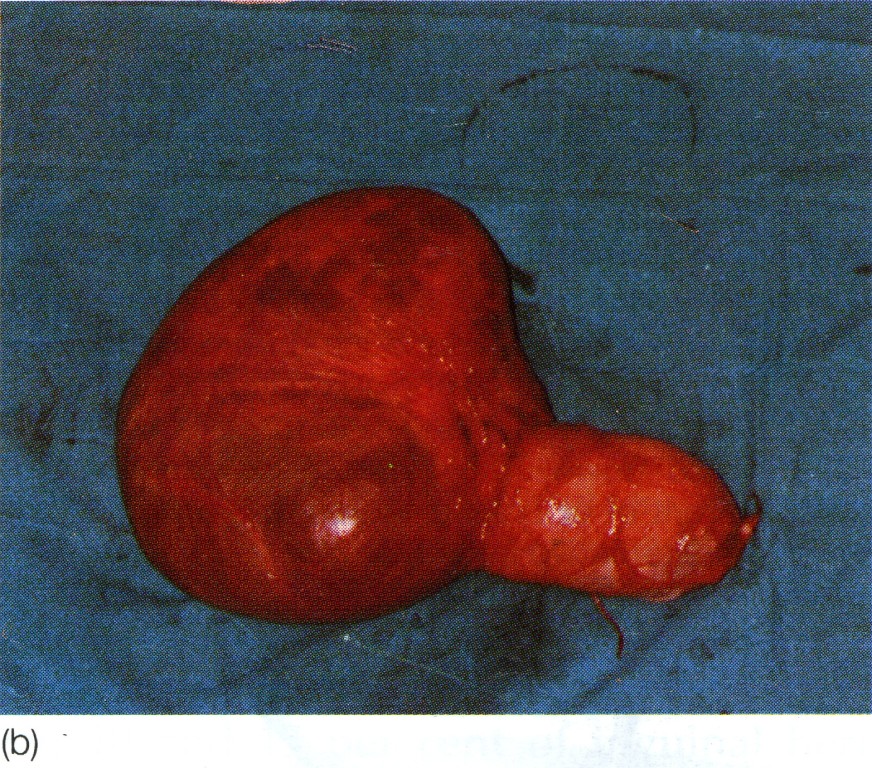
Fig. 7. Large transilluminant cystic swelling present in right lower abdomen, extending down the inguinal canal into the scrotum, (a) Lesion being removed; (b) excised specimen. It is not possible to distinguish between a complex scrotal hernia, a hydrocele of the cord and a vaginal hydrocele in such a case before exposing the anatomy; in this case the lesion was an abdominoscrotal hydrocele (hydrocele-en-bisac). (Drs D. Pratep and R. Sahai, Jhansi, India.)
Treatment of indirect inguinal hernia
Operative treatment. Operation is the treatment of choice. It must be remembered that patients who have a bad cough from chronic bronchitis should not necessarily be denied operation, for these are the very people who are in danger of getting a strangulated hernia. As these patients are often elderly, the surgeon should consider giving extra strength to the repair by a synchronous orchidectomy and full closure of the internal ring. In adults, local, epidural or spinal, as well as general anaesthesia can be used.
Inguinal herniotomy is the basic operation which entails dissecting out and opening the hernial sac, reducing any contents and then transfixing the neck of the sac and removing the remainder. It is employed either by itself or as the first step in a repair procedure (herniorrhaphy). By itself it is sufficient for the treatment of hernia in infants, adolescents and young fit adults who have good inguinal musculature. In fact, any attempts at repair in such cases are meddlesome and do more harm than good.
In infants it is not necessary to open the canal, as the internal and external rings are superimposed. Excellent results are obtained. The operation should be done in the morning and the child allowed home in the evening. Usually there is no need for the child to stay in hospital and 'the best nurse is the mother'. Although bilateral inguinal hernias are present in 30 per cent of cases of male infantile indirect inguinal hernias, it is probably not justified to explore the other side of cases of unilateral presentation, most of whom do not have a hernia on the normal side. In female infants, in whom bilateral sacs are very common, bilateral exploration is justified.
Herniotomy and repair (herniorrhapy). This operation consists of (1) excision of the hernial sac (above), plus (2) repair of the stretched internal inguinal ring and the transversalis fascia, and (3) further reinforcement of the posterior wall of the inguinal canal. Stages (2) and (3) must be achieved without tension, usually by 'darning' with a monofilament suture material such as polypropylene. Fascial flaps, or synthetic mesh implants, are employed when the deficiency of the posterior wall is extensive.
Operative procedures
General principles. Excision of the hernial sac (adult herniotomy).
Before the skin incision in large inguinoscrotal hernias, the usual antiseptic preparation of the skin should not be extended to the perneal aspect of the scrotum, for, by so doing, severe bacterial contamination of the operation site is likely. The operation approach should be confined to the anterior inguinal and scrotal aspects. An incision is made in the skin and subcutaneous tissues 1.25 cm above and parallel to the medial two-thirds of the inguinal ligament. In large irreducible herniae the incision is extended into the upper part of the scrotum. After dividing the superficial fascia and securing complete haemostasis, the external oblique aponeurosis and the superficial inguinal ring are identified. The external oblique aponeurosis is incised in the line of its fibres, and the structures beneath are carefully separated from its deep surface before completing the incision through the superficial inguinal ring. In this way the ilioinguinal nerve is safeguarded. With the inguinal canal thus opened, the upper leaf of the external oblique aponeurosis is separated by blunt dissection from the internal oblique. The lower leaf is likewise dissected until the inner aspect of the inguinal ligament is seen. The cremasteric muscle fibres are divided longitudinally to open up the subcremasteric space and display the spermatic cord, which is then lifted out.
Excision of the sac. The indirect sac is easily distinguished as a pearly white structure lying on the upper side of the cord and, when the internal spermatic fascia has been incised longitudinally, it can be dissected out and then opened between haemostats.
Variations in dissection. If the sac is small (e.g. bubonocele) it can be freed in toto. If it is of the long funicular or scrotal type, or is extremely thickened and adherent, the fundus need not be sought. The sac is freed and divided in the inguinal canal. Care must be taken to avoid damage to the vas and the spermatic artery, including the blood supply to the epididymis.
An adherent sac can be separated from the cord by first injecting saline under the posterior wall from within. A similar tactic is used when dissecting the gossamer sac of infants and children.
Reduction of contents. Intestine or omentum is returned to the peritoneal cavity. Omentum is often adherent to neck or fundus of the sac; if to the neck, it is freed, and if to the fundus of a large sac, it may be transfixed, ligated and cut across at a suitable site. The distal part of omentum, like the distal part of a large scrotal sac, can be left in situ (the fundus should not, however, be ligated).
Isolation and ligation of the neck of the sac. Whatever type of sac is encountered, it is necessary to free its neck by blunt and gauze dissection until the parietal peritoneum can be seen on all sides. Only when the extraperitoneal fat is encountered and the inferior epigastric vessels are seen on the medial side has the dissection reached the required limit. If it has not been done already, the sac is opened. The finger is passed through the mouth of the sac in order to make sure that no bowel or omentum is adherent. The neck of the sac is transfixed and ligated as high as possible, and the sac is excised 1.25 cm below the ligature.
Repair of the transversalis fascia and the internal ring. When the internal ring is weak and stretched, and the transversalis fascia is bulging, the repair should include the Lytle method of repairing and narrowing the ring with lateral displacement of the cord (Fig. 8), or the Toronto (Shouldice) method, whereby the ring and fascia are incised and carefully separated from the deep inferior epigastric vessels and extraperitoneal fat before an overlapping repair ('double breasting') of the lower flap behind the upper flap is effected. This repair using monofilament continuous suturing or darning materials (e.g. polypropylene, poly amide, wire) and avoiding tension is the basis for obtaining good results.
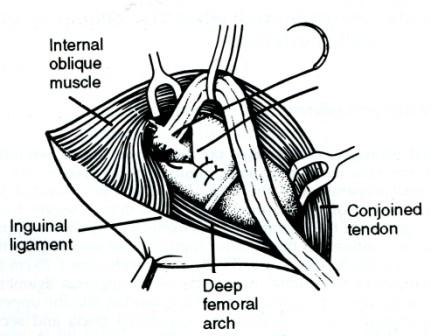
Fig. 8. The Lytle method of repair of the stretched internal inguinal ring which should be narrowed to admit the tip of the little finger. Lateral displacement of the cord is often advantageous, (fitter F.S.A. Dora/7, FRCS, Bromsgrove, England.)
Reinforcement of the posterior inguinal wall is achieved by approximating without tension the tendinous and aponeurotic part of the conjoined muscle to the pubic tubercle (and, according to some, to Astley Cooper's iliopectineal ligament) and to the undersurface of the inguinal ligament. Care is taken not to pick up the same tendinous bundle for each suture. Suturing of muscle bundles is of no value. The suturing method includes a choice between:
• simple interrupted stitches (Bassini type);
• darning (Fig. 9);
• a rectus-relaxing incision (Halsted-Tanner) combined with either of the above;
• a Dacron or other mesh implant (Fig. 10).
Other techniques include overlapping the external oblique behind the cord (making it lie subcutaneously). Special care is needed to avoid excessive narrowing of the new external ring which would jeopardise the vascular supply to, and the venous return from, the testis.
F ig.
9. The first layer of the two-layer darn of the posterior wall of the
inguinal canal. The darning is conducted from the pubic tubercle up
to and above the deep inguinal ring and back to the starting point.
The
darning is kept fairly loose, and it forms a lattice upon which
fibrous tissue is laid down. The external oblique aponeurosis is
reunited either in front of or behind the cord. Meticulous asepsis is
essential. Inset an alternative lattice darn.
ig.
9. The first layer of the two-layer darn of the posterior wall of the
inguinal canal. The darning is conducted from the pubic tubercle up
to and above the deep inguinal ring and back to the starting point.
The
darning is kept fairly loose, and it forms a lattice upon which
fibrous tissue is laid down. The external oblique aponeurosis is
reunited either in front of or behind the cord. Meticulous asepsis is
essential. Inset an alternative lattice darn.
F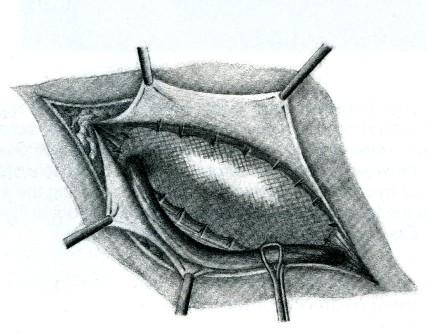 ig.
10. Dacron mesh reinforcement. The mesh is attached to the inguinal
ligament, the coverings of the pubis, the rectus sheath and the
conjoined aponeurosis and tendon. It is also fashioned by a slot
laterally above and below the cord at the internal ring.
ig.
10. Dacron mesh reinforcement. The mesh is attached to the inguinal
ligament, the coverings of the pubis, the rectus sheath and the
conjoined aponeurosis and tendon. It is also fashioned by a slot
laterally above and below the cord at the internal ring.
Specific types of repair. Herniotomy. This is particularly recommended for infants and young, athletic men (see above).
Inguinal repairs. The Shouldice (Toronto) repair is recommended for most indirect inguinal hernias. For very large hernias, a mesh may need to be used. In elderly men, orchidectomy should be considered. Extraperitoneal repairs are useful when the abdominal wall is very weak and recurrence is to be expected. Bilateral hernias, or combined inguinal and femoral hernias, and above all, recurrent hernias are often treated best by this method. Laparoscopic repair does not permit repair of the musculoaponeurotic structures of the canal although a mesh can be put in. It is still an unproven technique which should be used for special indications, e.g. serious risk of cardiorespiratory complications.
A satisfactory repair of an inguinal hernia can be done under local anaesthetic and should have a nil mortality rate and a recurrence rate of 1 per cent.
Sepsis is the main complication of inguinal hernia repair and can be avoided by careful technique (see below).
Completion of operation. If possible the cremasteric muscle should be reconstituted: the external oblique is directly sutured or overlapped leaving a new external ring which will accommodate the tip of the little finger.
A truss is used when operation is contraindicated because of cardiac, pulmonary, or other systemic disease, or when operation is refused. The hernia must be reducible. A rat-tailed spring truss with a perineal band to prevent the truss slipping will, with due care and attention, control a small or moderate-sized inguinal hernia. A truss must be worn continuously during waking hours, kept clean, and in proper repair, and renewed when it shows signs of wear. It must be applied before the patient gets up and while the hernia is reduced. A properly fitting truss must control the hernia when the patient stands with his legs wide apart, stoops, and coughs violently. If it does not it is a menace, for it increases the risk of strangulation.
Infants and trusses. Special inflatable rubber trusses, formerly popular for the control of an infant's hernia, are rarely used. Provided urgent admission is not required for sudden irreducibility, the parents are advised to wait for operation until the infant is at least 3 months old.
Direct inguinal hernia
Between 10 and 15 per cent of inguinal hernias are direct. Over half of these are bilateral.
A direct inguinal hernia is always acquired. The sac passes through a weakness or defect of the transversalis fascia in the posterior wall of the inguinal canal. In some cases the defect is small and closely related to the insertion of the conjoint tendon, while in others there is a generalised bulge. Often the patient has poor lower abdominal musculature, as shown by the presence of elongated bulgings (Malgaigne's bulges). Women practically never develop a direct inguinal hernia (Brown). Predisposing factors are a chronic cough, straining, and heavy work. Damage to the ilioinguinal nerve (e.g. by previous appendicectomy) is another known cause.
Direct hernias sometimes attain a large size and descend into the scrotum (Fig. 11) but this is rare. In contradistinction to an oblique inguinal hernia, a direct inguinal hernia lies behind the spermatic cord. The sac is often smaller than the hernial mass would indicate, the protruding mass mainly consisting of extraperi-toneal fat. As the neck of the sac is wide, direct inguinal hernias rarely strangulate.
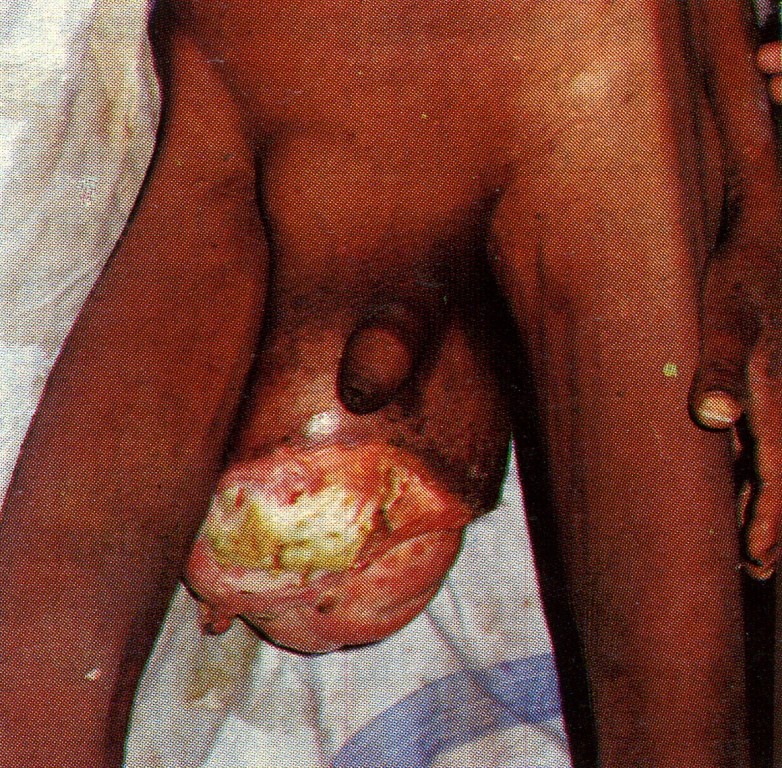
Fig. 11. Shows a huge inguinal hernia (direct) which has descended into the scrotum, and the overlying skin has become gangrenous and sloughed away. (Dr Anupam Rai, Jabalpur, India.)
Funicular direct inguinal hernia (syn. prevesical hernia) is a narrow-necked hernia of prevesical fat and a portion of the bladder that occurs through a small oval defect in the medial part of the conjoined tendon just above the pubic tubercle. It occurs principally in elderly males, and occasionally it becomes strangulated. Unless there are definite centra-indications, operation should always be advised.
Dual (syn. saddle-bag; pantaloon) hernia. Here there are two sacs which straddle the inferior epigastric artery, one sac being medial and the other lateral to this vessel. The condition is not a rarity, and is a cause of recurrence, one of the sacs having been overlooked at the time of operation.
Operation for direct hernia. The principles of repair of direct hernia are the same as those of an indirect hernia with the exception that the hernial sac need not be removed, for after it has been dissected free from surrounding structures it is inverted into the abdomen and the transversalis fascia repaired in front of it. Some form of reconstruction of the posterior wall of the inguinal canal is necessary, Polypropylene darns, and Dacron mesh insertions are popular (Figs. 9 and 10).
Complications of herniorrhaphy are:
• wound sepsis (especially due to faulty technique);
• haematoma;
• lymphocele (commoner after operations for femoral hernia);
• wound sinus (especially when foreign tissue is used for the repair);
• division of spermatic cord (especially in infantile hernia operation);
• testicular ischaemia (especially after large or recurrent hernia repairs);
• testicular atrophy;
• hydrocele;
• nerve entrapment
- pain
- parasthesia or numbness;
• recurrence (especially after operations for large hernias in elderly males or sepsis);
• general
- retention of urine
- respiratory complications
- thromboembolic complications.
Strangulated inguinal hernia
(Pathological and clinical features are described earlier in this chapter.)
Strangulation of an inguinal hernia occurs at any time during life, and in both sexes. Oblique inguinal hernias strangulate commonly; the direct variety but rarely owing to the wide neck of the sac. Sometimes a hernia strangulates on the first occasion that it descends; more often strangulation occurs in patients who have worn a truss for a long time, and in those with a partially reducible or irreducible hernia. Strangulation occurs most frequently in large hernias in elderly patients, which is an excellent reason for an aggressive approach to hernia repair in younger patients.
In order of frequency, the constricting agent is:
1. the neck of the sac;
2. the external abdominal ring (in children especially);
3. rarely adhesions within the sac.
Contents. Usually small intestine is involved in the strangulation; the next most frequent content is omentum; sometimes both are implicated. For large intestine to become strangulated in an inguinal hernia is of the utmost rarity, even when the hernia is of the sliding variety.
Strangulation during infancy. The incidence of strangulation is 4 per cent (Gross), and the ratio of females to males is 5:1. More frequently the hernia is irreducible but not strangulated. In most cases of inguinal hernia occurring in female content of the sac is an ovary, or an ovary plus its Fallopian tube.
Treatment of strangulated inguinal hernia. The treatment of strangulated hernia is by emergency operation. (The danger is in the delay, not in the operation' - Astley Cooper.)
If dehydration and collapse are present, intravenous fluid replacement and gastric aspiration for 1-3 hours are invaluable. It is absolutely essential to make sure that the stomach is emptied just before commencing the anaesthetic. The passing of a large-bore stomach tube is the best way of preventing vomiting, drowning, and cardiac arrest during the induction. The bladder must also be emptied, if necessary by a catheter. Suitable broad-spectrum antimicrobials are i.v.
Inguinal herniotomy for strangulation. An incision is made over the most prominent part of the swelling. The external oblique aponeurosis is exposed, and the sac, with its coverings, is seen issuing from the superficial inguinal ring. In all but very large hernias it is possible to deliver the body and fundus of the sac together with its coverings and (in the male) the testis onto the surface. Each layer covering the anterior surface of the body of the sac near the fundus is incised, and if possible it is stripped off the sac. The sac is then incised, the fluid therein is mopped up or aspirated very thoroughly, for it can be highly infected. The external oblique aponeurosis and the superficial inguinal ring are divided. Returning to the sac, a finger is passed into the opening, and employing the finger as a guide, the sac is slit along its length. If the lies at the superficial inguinal ring or in the inguinal canal, it is readily divided by this procedure. When the constricting agent is at the deep inguinal ring, by applying haemostats to the cut edge of the neck of the sac and drawing them downwards, and at the same time retracting the internal oblique upwards, it may be possible to continue slitting up the sac over the finger beyond the point of constriction. When the constriction is too tight to admit a finger, a grooved director is inserted and the neck of the sac is divided with a hernia knife in an upward and inward direction, i.e. parallel to the inferior epigastric artery, under vision. Once the constricting agent has been divided, the strangulated contents can be drawn down. Devitalised omentum is excised after being securely ligated, by interlocking stitches if bulky. Viable intestine is returned to the peritoneal cavity. Doubtfully viable and gangrenous intestine is dealt with. If the hernial sac is of moderate size and can be separated easily from its coverings, it is excised and closed by a purse-string suture. When the sac is large and adherent, much time is saved by cutting across the sac. Having tied or sutured the neck of the sac a repair can be made if the condition of the patient permits, but synthetic implants should be avoided because of the risks of infection.
Conservative measures. These are only indicated in infants. Children are given a sedative and then slung to a Balkan beam1 or to the bedrail by their feet (the judgment of Solomon position) for no longer than 3 hours. In 75 per cent of cases reduction is effected and there appears to be no danger of gangrenous intestine being reduced (Irvine Smith). Manual reduction of irreducible and strangulated hernias under sedation is permissible in infants in whom the risk of intestinal gangrene appears to be almost nonexistent for many hours. For all other cases, vigorous manipulation (taxis) has no place in modern surgery, and is mentioned only to be condemned. Its dangers include:
• Contusion or rupture of the intestinal wall.
• Reduction-en-masse. The sac together with its contents, is pushed forcibly back into the abdomen; and as the bowel will still be strangulated by the neck of the sac, the symptoms are in no way relieved` (Treves).
• Reduction into a loculus of the sac.
• The sac may rupture at its neck and its contents are reduced, not into the peritoneal cavity, but extraperitoneally.
Maydl's hernia (syn. hernia-in-W) is rare. The strangulated loop of the W lies within the abdomen, thus local tenderness over the hernia is not marked. At operation two comparatively normal-looking loops of intestine are present in the sac. After the obstruction has been relieved, the strangulated loop will become apparent if traction is exerted on the middle of the loops occupying the sac.
Inguinal hernia recurrence
Only by using a meticulous technique, principally concentrating on the repair of the transversalis fascia and the internal ring, can a recurrence rate of less than 2 per cent be achieved. Recurrences through the repair tend to occur within 2 years. In a few cases false recurrences occur, i.e. another type of hernia occurs -direct after indirect, femoral after direct or indirect. To the patient it is a recurrence!
The spermatic cord as a barrier to effective closure of the inguinal canal. In the elderly patient, removal of the testis and cord aids in an effective repair in the case of recurrent inguinal hernia, sliding hernia, and some large direct hernias. The signed permission of the patient must always be obtained beforehand.
Sliding hernia (syn. hernie-en-glissade) (Fig. 12)
As a result of slipping of the posterior parietal peritoneum on the underlying retroperitoneal structures, the posterior wall of the sac is not formed of peritoneum alone, but by the sigmoid colon and its mesentery on the left, the caecum on the right and, sometimes, on either side by a portion of the bladder. It should be clearly understood that the caecum, appendix, or a portion of the colon wholly within a hernial sac does not constitute a sliding hernia. A small-bowel sliding hernia occurs once in 2000 cases; a sacless sliding hernia once in 8000 cases.
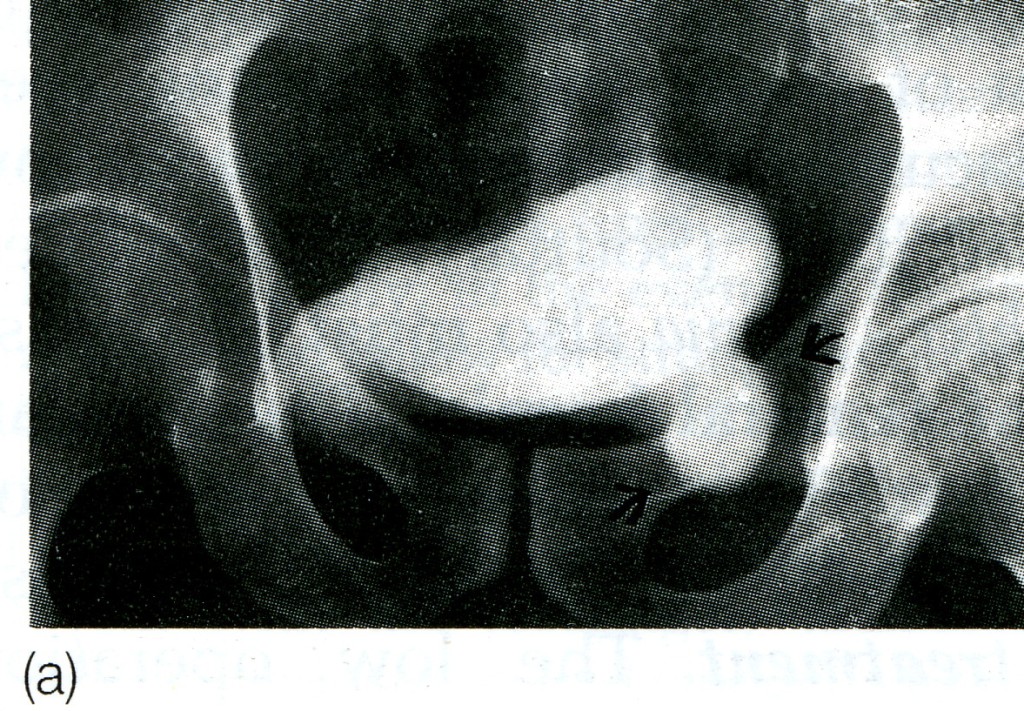
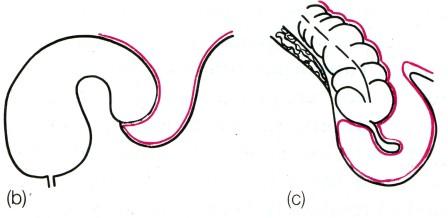
Fig. 12. Sliding hernia, (a) Cystogram showing bladder involving a left inguinal hernia, (b) Diagram of the same, (c) Caecum and appendix in right sliding hernia.
Clinical features. A sliding hernia occurs almost exclusively in males. Five out of six sliding hernias are situated on the left side; bilateral sliding hernias are exceedingly rare. The patient is nearly always over 40, the incidence rising with the weight of years. There are no clinical findings that are pathognomonic of a sliding hernia, but it should be suspected in every large globular inguinal hernia descending well into the scrotum. Large intestine is commonly present in a sliding hernia (or caecum and appendix in a right-sided case).
Occasionally large intestine is strangulated in a sliding hernia; more often nonstrangulated large intestine is present behind the sac containing strangulated small intestine.
Treatment. A sliding hernia is impossible to control with a truss, and as a rule the hernia is a cause of considerable discomfort. Consequently operation is indicated, and the results generally are good.
Operation. It is unnecessary to remove any of the sliding hernial sac provided it is freed completely from the cord and the abdominal wall, and that it is replaced deep to the repaired fascia transversalis. In many instances it is desirable to perform orchiectomy in order to effect a secure repair. No attempt should be made to dissect the caecum or colon free from the peritoneum under the impression that these are adhesions, in which case peritonitis or a faecal fistula resulting from necrosis of a devascularised portion of the bowel may occur. This is specially liable to occur on the left side, as vessels in the mesocolon may be injured.
Femoral hernia
Femoral hernia is the third most common type of hernia (incisional hernia comes second). It accounts for about 20 per cent of hernias in women, and 5 per cent in men. The overriding importance of femoral hernia lies in the facts that it cannot be controlled by a truss, and that of all hernias it is the most liable to become strangulated mainly because of the narrowness of the neck of the sac and the rigidity of the femoral ring.
Surgical anatomy. The femoral canal occupies the most medial compartment of the femoral sheath, and it extends from the femoral ring above to the saphenous opening below. It is 1.25 cm long, and 1.25 cm wide at its base, which is directed upwards. The femoral canal contains fat, lymphatic vessels, and the lymph node of Cloquet. It is closed above by the septum crurale, a condensation of extraperitoneal tissue pierced by lymphatic vessels, and below by the cribriform fascia.
The femoral ring is bounded:
• anteriorly by the inguinal ligament;
• posteriorly by Astley Cooper's (iliopectineal) ligament, the pubic bone, and the fascia over the pectineus muscle;
• medially by the concave knife-like edge of Gimbernat's (lacunar) ligament, which is also prolonged along the iliopectineal line as Astley Cooper's ligament;
• laterally by a thin septum separating it from the femoral vein.
Sex incidence. The female to male ratio is about 2:1, but it is interesting that whereas the female patients are frequently elderly, the male patients are usually between 30 and 45 years. The condition is more prevalent in women who have borne children than in nulliparas. The broader female pelvis also predisposes to the condition.
Pathology. A hernia passing down the femoral canal descends vertically as far as the saphenous opening. While it is confined to the inelastic walls of the femoral canal the hernia is necessarily narrow, but once it escapes through the saphenous opening into the loose areolar tissue of the groin, it expands, sometimes considerably. A fully distended femoral hernia assumes the shape of a retort, and its bulbous extremity may be above the inguinal ligament. By the time the contents have pursued so tortuous a path they are usually irreducible and apt to strangulate, a circumstance which is also favoured by the rigidity of the surrounds of the femoral ring.
Clinical features. Femoral hernia is rare before puberty. Between 20 and 40 years of age the prevalence rises, and continues to old age. The right side (Fig. 13) is affected twice as often as the left, and in 20 per cent of cases the condition is bilateral. The symptoms to which a femoral hernia gives rise are less pronounced than those of an inguinal hernia; indeed, a small femoral hernia may be unnoticed by the patient or disregarded for years, until perhaps the day it strangulates. Adherence of greater omentum sometimes causes a dragging pain. Rarely, a large sac is present.
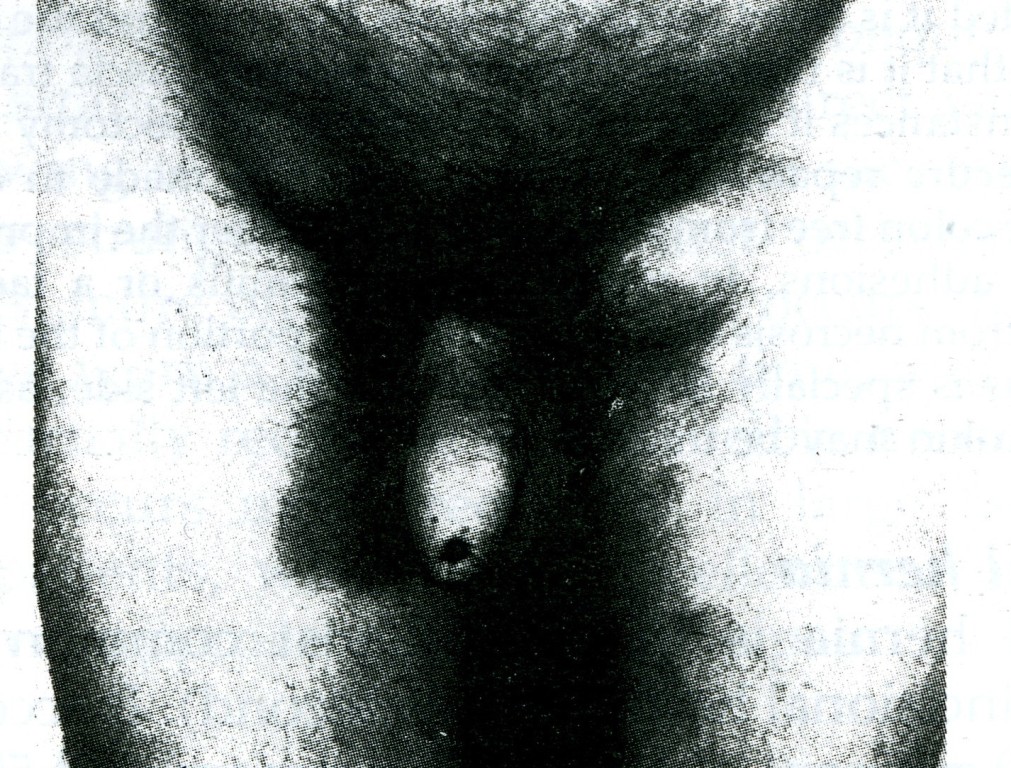
Fig. 13. The patient has a left inguinal and a right femoral hernia (as in Fig. 14).
Differential diagnosis. A femoral hernia has to be distinguished from the following.
An inguinal hernia. An inguinal hernia lies above and medial to the medial end of the inguinal ligament at its attachment to the pubic tubercle. The femoral hernia lies below this (Fig. 14). Occasionally the fundus of a femoral hernia sac overlies the inguinal ligament.
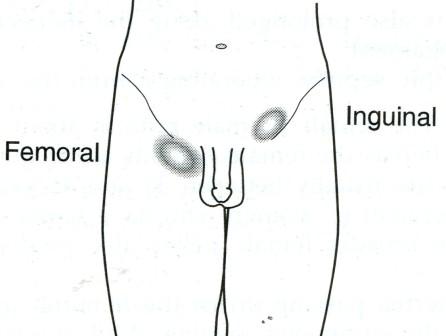
Fig. 14. The essentials of differential diagnosis between a femoral and an inguinal hernia (see text) (as in Fig. 13).
A saphena varix. A saphena varix is a saccular enlargement of the termination of the long saphenous vein and it is usually accompanied by other signs of varicose veins. The swelling disappears completely when the patient lies down, while a femoral hernia sac usually is still palpable. In both there is an impulse on coughing. A saphena varix will, however, impart a fluid thrill to the examining fingers when the patient coughs, or when the saphenous vein below the varix is tapped with the fingers of the other hand. Sometimes a venous hum can be heard when a stethoscope is applied over a saphena varix.
An enlarged femoral lymph node. If there are other enlarged lymph nodes in the region the diagnosis is tolerably simple, but when Cloquefs lymph node alone is affected the diagnosis may be impossible unless there is a clue, such as an infected wound or abrasion on the corresponding limb or on the perineum. Doubt should be removed by immediate surgery.
Lipoma.
A femoral aneurysm.
A psoas abscess. There is often a suprainguinal fluctuating swelling - an iliac abscess - which communicates with the femoral swelling in question. Examination of the spine and a radiograph will settle the diagnosis.
A distended psoas bursa. The swelling diminishes when the hip is flexed, and osteoarthrosis of the hip is present.
Rupture of the adductor longus with haematoma.
Hydrocele of a femoral hernial sac. The neck of the sac becomes plugged with omentum or by adhesions, and a hydrocele of the hernial sac results.
Laugier's femoral hernia is a hernia through a gap in the lacunar (Gimbernafs) ligament. The diagnosis is based on unusual medial position of a small femoral hernial sac. Nearly always the hernia has strangulated.
Narath's femoral hernia occurs only in patients with congenital dislocation of the hip and is due to lateral displacement of the psoas muscle. The hernia lies hidden behind the femoral vessels.
Cloquet's hernia is one in which the sac lies under the fascia covering the pectineus muscle. Strangulation is likely. The sac may coexist with the usual type of femoral sac.
Strangulated femoral hernia. A femoral hernia strangulates frequently and gangrene develops rapidly. This is accounted for by the narrow, unyielding femoral ring. In 40 per cent of cases the obstructing agent is not Gimbernafs (lacunar) ligament but the narrow neck of the femoral sac itself (Souttar). The frequent occurrence of a Richter's hernia also must be stressed.
Treatment of femoral hernia. The constant risk of strangulation is sufficient reason for urging operation. A truss is contraindicated because of this risk.
Operative treatment. The low operation (Lock-wood), the high operation (McEvedy), and the inguinal operation (Lotheissen) all have their advocates, with special controversy surrounding possible surgical laceration of an abnormal origin of the obturator artery from the inferior epigastric artery during a below-ligament (low) approach. The abnormal artery descends closely related to the posterior surface of Gimbernafs ligament. In all cases the stomach and bladder must be emptied immediately before commencing the operation.
The low operation (Lockwood). The sac is dissected out below the inguinal ligament via a groin-crease incision. It is essential to peel off all the anatomical layers which cover the sac. These are often thick and fatty. After dealing with the contents (e.g. freeing adherent omentum) the neck of the sac is pulled down, ligated as high as possible and allowed to retract through the femoral canal. The canal is closed by suturing the inguinal ligament to the iliopectineal line using unabsorbable sutures. This technique carries the highest (although still rare) possibility for damage to an abnormal artery. It is not recommended for use in cases of strangulation as exposure and control of the prolapsing intestine is limited through this approach.
The high (McEvedy) operation. A vertical incision is made over the femoral canal and continued upwards above the inguinal ligament. Through the lower part of the incision the sac is dissected out. The upper part of the incision exposes the inguinal ligament and the rectus sheath. The superficial inguinal ring is identified, and an incision 2.5 cm above the ring and parallel to the outer border of the rectus muscle is deepened until the extraperitoneal space is found. By gauze dissection in this space the hernial sac entering the femoral canal can be easily identified. Should the sac be empty and small, it can be drawn upwards; if it is large, the fundus is opened below, and its contents, if any, dealt with appropriately before delivering the sac upwards from its canal. The sac is then freed from the extraperitoneal tissue and its neck is ligated. An excellent view of the iliopectineal ligament is obtained and the conjoined tendon is sutured to it with non-absorbable sutures. Strangulation. An advantage of the operation is that, if resection of intestine is required, ample room can be obtained by opening the peritoneum. The disadvantage of the operation is that, if infection occurs, incisional hernia is not very unusual. This technique protects the origin of an abnormal artery.
Lotheissen's operation. The inguinal canal is opened as for inguinal herniorrhaphy. The transversalis fascia is incised to the medial side of the epigastric vessels and the opening is enlarged. The peritoneum is now in view; one must be certain that it is the peritoneum and not the bladder or a diverticulum thereof. The peritoneum is picked up with dissecting forceps, and incised. It is now possible to ascertain if any intraperitoneal structure is entering the femoral sac. Should the sac be empty, haemostats are placed upon the edges of the opening into the peritoneum, and by gauze dissection the sac is withdrawn from the femoral canal. An empty sac can be delivered easily; in the event of the sac being occupied, the technique described below for strangulation should be followed. This technique allows easy control of an abnormal artery that is damaged at surgery. The conjoined tendon is sutured to the iliopectineal line to form a shutter. While protecting the external iliac/femoral vein with the forefinger, unabsorbable sutures are passed through the periosteum and Cooper's ligament overlying the iliopectineal line. The retractor having been removed, the long ends of the sutures are passed from within outwards, through the conjoined tendon, and tied, thus approximating the conjoined tendon to the iliopectineal line. If there is any tension, a Tanner's slide will facilitate this step. The incised external oblique is sutured.
Strangulation. Modified Lotheissen operation. As soon as the external oblique has been exposed, the inferior margin of the wound is retracted strongly, thereby displaying the swelling. The coverings of the sac are incised and peeled off, until the sac, dark from contained bloodstained fluid, is apparent. The sac is incised, and the fluid that escapes is mopped up with great care. The retractor is removed and the operation is continued above the inguinal ligament in the same way as described already. Once the peritoneum has been opened above the inguinal ligament, one can see exactly what is entering the sac. Should the obstruction lie in a narrow neck of the sac, the beak of a haemostat is insinuated, and with great care the neck is stretched. (An abnormal obturator artery is present either on the medial or the lateral side of the neck of the sac in 28 per cent of cases.) The contents of the sac are delivered, and dealt with. Sometimes, in order to facilitate reduction of the hernial contents, it becomes necessary to divide or digitally to avulse part of Gimbernafs ligament.
It must be re-emphasised that, throughout operations for the repair of a femoral hernia, on the lateral side, the external iliac/femoral vein must be protected, and on the medial side great care must be taken not to injure the bladder, particularly since a portion of the bladder may form part of the wall of the sac (a sliding femoral hernia).
Umbilical hernia
Exomphalos (syn. omphalocele) occurs once in every 6000 births; it is due to failure of all or part of the midgut to return to the coelom during early fetal life. Sometimes a large sac ruptures during birth. When the sac remains unruptured, it is semitranslucent (Fig. 15), and although very thin it consists of three layers - an outer layer of amniotic membrane, a middle layer of Wharton's jelly, and an inner
layer of peritoneum. There are two varieties of exomphalos:
Fig. 15. Exomphalos. The delicate sac ruptured soon afterwards.
Exomphalos minor. The sac is relatively small and to its summit is attached the umbilical cord. Inadvertently a loop of small intestine or a Meeker s diverticulum can be included in the ligature applied to the base of an umbilical cord containing this protrusion.
Exomphalos major. The umbilical cord is attached to the inferior aspect of the swelling, which contains small and large intestine, and nearly always a portion of the liver. Half the cases belong to this group.
Treatment. Exomphalos minor. It is necessary only to twist the cord, so as to reduce the contents of the sac through the narrow umbilical opening into the peritoneal cavity, and to retain them by firm strapping. Despite a seropurulent discharge on no account must the strapping be removed for fourteen days.
Exomphalos major. Operation within the first few hours of life is the only hope, otherwise the sac will burst. To prevent further distension of the contents of the sac, the infant should not be fed. A few newborn infants with a ruptured sac have survived following immediate operation and antibiotic therapy.
Operation. It must be realised that most of the contents of the sac have never been housed within the abdominal cavity; consequently that cavity is unduly small, and to attempt to replace the contents of the sac is like endeavouring to put 2 kg of sugar into a 1 kg bag - a feat that so often results in respiratory embarrassment, compromise of venous return, and possibly intestinal obstruction. It is necessary to create flaps of skin by undermining the subcutaneous tissue on either side, so that the flaps can be brought together over the sac. If necessary, relaxing incisions must be made in the loins to permit closure. For several days following the operation it is advisable to carry out aspiration through an indwelling gastric tube, to relieve or prevent distension. If the patient survives the construction of this protective cutaneous covering, repair of the hernia can be delayed for months, or even years. At the second operation, it is surprising to find that the peritoneum and the muscles can be drawn together and closed in layers.
Congenital umbilical hernia. Rarely, a fully developed umbilical hernia is present at birth presumably due to intrauterine epithelialis-ation of a small exomphalos.
Umbilical hernia of infants and children. This is a hernia through a weak umbilical scar. The ratio of males to females is 2:1. Most do not become obvious until the infant is several weeks old. The hernia is often symptomless, but increase in the size of the hernia on crying causes pain, which makes the infant cry the more. Small hernias are spherical; those that increase in size tend to assume a conical shape (Fig. 16) and are present apart from crying. Obstruction or strangulation below the age of three years is extremely uncommon.
Fig. 16. Infantile umbilical hernia.
Treatment. Conservative treatment by masterly inactivity is successful in about 93 per cent of cases. When the hernia is symptomless, reassurances of the parents is all that is necessary, for in a very high percentage of cases the hernia will be found to disappear spontaneously during the first few months of life. Cure may also be hastened by pulling the skin and abdominal musculature together by adhesive strapping placed across the abdomen.
Herniorrhaphy. In cases where masterly inactivity fails, operation is required, and it should be carried out, preferably, about the age of 2 years.
O peration.
In infants
a small curved incision
is made
immediately below the umbilicus. The skin cicatrix is
dissected
upwards, and the neck of the sac is isolated. After ensuring that the
sac is empty of contents, it is either inverted into the abdomen or
it is ligated by transfixion and excised. The defect in the linea
alba is closed with two unabsorbable sutures.
peration.
In infants
a small curved incision
is made
immediately below the umbilicus. The skin cicatrix is
dissected
upwards, and the neck of the sac is isolated. After ensuring that the
sac is empty of contents, it is either inverted into the abdomen or
it is ligated by transfixion and excised. The defect in the linea
alba is closed with two unabsorbable sutures.
Fig. 17. A large paraumbilical hernia.
Paraumbilical hernia of adults (syn. supra- or infraumbilical hernia). In adults the hernia does not occur through the umbilical scar. It is a protrusion through the linea alba just above or sometimes just below the umbilicus. As it enlarges, it becomes rounded or oval in shape with a tendency to sag downwards. Paraumbilical hernias can become very large (Fig. 17). The neck of the sac is often remarkably narrow as compared with the size of the sac and the volume of its contents, which consist of greater omentum often accompanied by small intestine and, alternatively or in addition, a portion of the transverse colon. In long-standing cases the sac sometimes becomes loculated due to adherence of omentum to its fundus.
Clinical features. Women are affected five times more frequently than men. The patient is usually corpulent and between the ages of 35 and 50. Increasing obesity, with flabbiness of the abdominal muscles, and repeated pregnancy are important antecedents. These hernias soon become irreducible because of omental adhesions within the sac. A large umbilical hernia causes a local dragging pain by its weight. Gastrointestinal symptoms are common and are probably due to traction on the stomach or transverse colon. Often there are transient attacks of intestinal colic due to subacute intestinal obstruction. In long-standing cases, intertrigo of the adjacent surfaces of the skin is a troublesome complication.
Treatment. Untreated, the hernia increases in size, and more and more of its contents become irreducible. Eventually, strangulation may occur. Therefore without undue delay operation should be advised in nearly all cases. If the patient is obese and the hernia is symptomless, operation can be postponed with advantage until weight has been reduced. When small, the deficiency can be closed by a simple repair using interrupted unabsorbable sutures: for larger hernias, a Mayo technique is advisable (Fig. 18).
Fig. 18. Mayo's operation for umbilical hernia. Interrupted sutures to provide an overlap are first inserted. Inset The overlap has been made and completed with a continuous suture. It is important to denude the area of fat before stitching the flap in position.
Mayo's operation. A transverse elliptical incision is made around the umbilicus. The subcutaneous tissues are dissected off the rectus sheath to expose the neck of the sac. The neck is incised to expose the contents. Intestine is returned to the abdomen. Any adherent omentum is freed, and ligated by transfixion if it is bleeding. Excess adherent omentum can be removed with the sac if necessary. The sac is then removed and the peritoneum of the neck closed with catgut. The aponeurosis on both sides of the umbilical ring is incised transversely for 2.5 cm or more - sufficiently to allow an overlap of 5 to 7.5 cm. Three to five mattress sutures of fine, unabsorbable material are then inserted into the aponeurosis as shown in Fig. 18. When this row of mattress sutures has been tied, the overlapping upper margin is stitched to the sheath of the rectus abdominis and the midline aponeurosis. In fat patients, who ooze blood and liquid fat, a fine suction drain is provided at each end of the wound. The subcutaneous fat and skin are then approximated with deep sutures. If the patient has a tendency to bronchitis, it is wise to prescribe antibiotic therapy and breathing exercises.
Additional lipectomy. In patients with a paraumbilical hernia associated with a large, pendulous, fat-laden abdominal wall the operation can, with great advantage, be combined with lipectomy by fashioning the incisions to embrace a larger area of the fat-laden superficial layers of the abdominal wall. Meticulous haemostasis is essential.
Strangulation is a frequent complication of a large paraumbilical hernia in adults. Owing to the narrow neck and the fibrous edge of the linea alba, gangrene is liable to supervene unless early operation is carried out. Also it should be remembered that in large hernias, the presence of loculi may result in a strangulated knuckle of the bowel in one part of an otherwise soft and nontender hernia.
Operation. In early cases the operation does not differ from that for nonstrangulated cases. Gangrenous contents are dealt with as in other situations. If a portion of the transverse colon is gangrenous, it should be exteriorised by the Paul-Mikulicz method and the gangrenous portion excised. If the ring is large enough to transmit the colon unhampered, it is left alone; otherwise it is enlarged. It is important that the small intestine be thoroughly scrutinised as a small loop may have been trapped and slipped back when the constriction was relieved. If nonviable gut is overlooked, peritonitis quietly supervenes, and the symptoms are ascribed to 'postoperative discomfort'. The condition of the patient steadily deteriorates before succumbing a few days later (Franklin).
Epigastric hernia
A midline epigastric hernia (syn. fatty hernia of the linea alba) occurs through the linea alba anywhere between the xiphoid process and the umbilicus, usually midway between these structures. Such a hernia commences as a protrusion of extraperitoneal fat through the linea alba, where the latter is pierced by a small blood vessel. Often more than one hernia is present.
A swelling the size of a pea consists of a protrusion of extraperitoneal fat only (fatty hernia of the linea alba). If the protrusion enlarges, it drags a pouch of peritoneum after it, and so becomes a true epigastric hernia. The mouth of the hernia is rarely large enough to permit a portion of hollow viscus to enter it; consequently, either the sac is empty or it contains a small portion of greater omentum.
Epigastric hernias are quite common in children, often accompanying divarication of the recti: both the divarication and the hernia undergo spontaneous cure in many cases.
It is probable that an epigastric hernia is the direct result of a sudden strain tearing the interlacing fibres of the linea alba. The patients are often manual workers between 30 and 45 years of age.
Clinical features.
• Symptomless. A small fatty hernia of the linea alba can be felt better than it can be seen, and may be symptomless, being discovered only in the course of routine abdominal palpation.
• Painful. Sometimes such a hernia gives rise to attacks of local pain (worse on physical exertion) and also tenderness to touch and tight clothing, possibly because the fatty contents become nipped sufficiently to produce partial strangulation.
• Referred pain (dyspeptic cases). It is not uncommon to find that the patient, who may not have noticed the hernia, complains of pain relating to digestion.
Treatment. If the hernia is giving rise to symptoms, operation should be undertaken. It is essential to mark the hernia before the anaesthetic is given as it may be impossible to locate the defect if the fatty protrusion retracts into the abdomen.
Operation. An adequate vertical or transverse incision is made over the swelling, exposing the linea alba. The protruding extraperi-toneal fat is cleared from the hernial orifice by gauze dissection. If the pedicle passing through the linea alba is slender, it is separated on all sides of the opening by blunt dissection. After ligating the pedicle, the small opening in the linea alba is closed by unabsorbable sutures. When a hernial sac is present it is opened and any contents reduced, after which the sac is excised before repairing the linea alba. Sometimes smaller protrusions of fat are found above and below the hernia. These should also be dealt with.
RARE EXTERNAL HERNIAS
Interparietal hernia (syn. interstitial hernia). An interparietal hernia has a hernial sac that passes between the layers of the anterior abdominal wall. The sac may be associated with, or communicate with, the sac of a concomitant inguinal or femoral hernia. Lack of knowledge of this condition is the cause of misdiagnosis and mismanagement.
Varieties:
• Properitoneal (20 per cent). Usually the sac takes the form of a diverticulum from a femoral or inguinal hernia.
• Intermuscular (60 per cent). The sac passes between the muscular layers of the anterior abdominal wall, usually between the external oblique and internal oblique muscles. The sac is nearly always bilocular, and is associated with an inguinal hernia.
• Inguinosuperficial (20 per cent). The sac expands beneath the superficial fascia of the abdominal wall or the thigh. This type is commonly associated with an incompletely descended testis.
Clinical features. The patients (mostly male) present with intestinal obstruction, due to obstruction or strangulation of the hernia. In the properitoneal variety, as no swelling is likely to be apparent, delays in diagnosis occur and consequently the mortality in this variety is high.
Treatment. Operation is imperative because of intestinal obstruction.
Spigelian hernia is a variety of interparietal hernia occurring commonly at the level of the arcuate line. The fundus of the sac, clothed by extraperitoneal fat, may lie beneath the internal oblique muscle, where it is virtually impalpable. More often it advances through that muscle and spreads out like a mushroom between the external and internal obliques and gives rise to a more evident swelling. The patient is often corpulent and usually over fifty years of age, men and women being affected equally. Typically, a soft, reducible mass will be encountered lateral to the rectus muscle and below the umbilicus. Because of the rigid fascia surrounding its neck, strangulation may occur.
Treatment. Operation. The external oblique aponeurosis is split. After isolating the sac, dealing with any contents, and ligating and excising it, the transversus, internal oblique, and external oblique muscles are repaired.
Lumbar hernia. Most primary lumbar hernias gain exit through the inferior lumbar triangle of Petit (Fig. 19), bounded below by the crest of the ilium, laterally by the external oblique, and medially by the latissimus dorsi. Some come through the superior lumbar triangle, which is bounded by the twelfth rib above, medially by the sacrospinalis, and laterally by the posterior border of the internal oblique. An extensive incisional lumbar hernia sometimes follows an operation upon an infected kidney.
Differential diagnosis. A lumbar hernia must be distinguished from:
• lipoma;
• a cold abscess pointing to this position;
• phantom hernia due to local muscular paralysis.
Lumbar phantom hernias can result from any interference with the nerve-supply of the affected muscles (e.g. poliomyelitis).
Fig. 19. Inferior lumbar hernia. It contained caecum, appendix and small bowel. Note filarial skin rash on buttocks. (V.J. Hartfield, formerly S.E. Nigeria.)
Treatment. A primary lumbar hernia, being small, is easily repaired. A postoperative lumbar hernia may be large, and the defect is impossible to repair unless fascial flaps are used. The area can be reinforced still further by stitching in place a piece of tantalum gauze or synthetic mesh.
Perineal hernia (syn. hernia through the pelvic floor) is rare.
• Postoperative hernia through a perineal scar may occur after excision of the rectum.
• Median sliding perineal hernia is a complete prolapse of the rectum (Chapter 53).
• Anterolateral perineal hernia occurs in women and presents as a swelling of the labium majus.
• Posterolateral perineal hernia, which passes through the levator ani to enter the ischiorectal fossa.
Treatment. Repair operations for the first two are described in Chapter 53. A combined operation is generally the most satisfactory for anterolateral or posterolateral hernias. The hernia is exposed by an incision directly over it. The sac is opened and its contents are reduced. The sac is cleared from surrounding structures and the wound is closed. With the patients in semi-Trendelenburg position, the abdomen is opened and the mouth of the sac is exposed. The sac is inverted, ligated, and excised, and the pelvic floor is repaired as adequately as possible.
Obturator hernia. The hernia, which passes through the obturator canal, occurs six times more frequently in women than in men. Most of the patients are over 60 years of age. The swelling is liable to be overlooked because it is covered by the pectineus. It seldom causes a definite swelling in Scarpa's triangle, but if the lower limb is flexed, abducted, and rotated outwards, sometimes the hernia becomes more apparent. The leg is usually kept in a semiflexed position and movement increases the pain. In more than 50 per cent of cases of strangulated obturator hernia pain is referred along the obturator nerve by its geniculate branch to the knee. On vaginal or rectal examination the hernia sometimes can be felt as a tender swelling in the region of the obturator foramen.
Cases of obturator hernia which present themselves have usually undergone strangulation, which is often of the Richter type.
Treatment consists of the following:
• Perform lower laparotomy (on the side of the lesion, if known). Confirm the diagnosis and then adopt full Trendelenburg's position.
• The constricting agent is the obturator fascia. Taking every precaution to avoid spilling infected fluid from the hernial sac into the peritoneal cavity, this fascia can be stretched to allow reduction by inserting suitable forceps through the gap in the fascia and opening the blades with care. If incision of the fascia is required, it is made parallel to the obturator vessels and nerve.
• The contents of the sac are dealt with.
• The broad ligament is stitched over the opening to prevent recurrence.
Gluteal and sciatic hernias. A gluteal hernia passes through the greater sciatic foramen, either above or below the piriformis. A sciatic hernia passes through the lesser sciatic foramen. Differential diagnosis must he made between these conditions and:
• a lipoma or fibrosarcoma beneath the gluteus maximus;
• a tuberculous abscess;
• a gluteal aneurysm.
All doubtful swellings in this situation should be explored by operation.
TESTS
1. Rgarding desmoid tumour which is noi correct
a) Often seen below the umbilicus
b) Unencapsulated
c) More common in women
d) Metastasis does not occur
e) Highly radiosensitive
2. Treatment of choice in desmoid tumours is -
a) Irradiation
b) Wide excision
c) Local excision
d) Local excision following radiation
3. Recurrent fibroma refers to Desmoid tumor arising in –
a) Uterus
b) Scar tissue
c) Ovary
d) Muscle
4. Meckel's diverticulum is -
a) Ductus venosus
b) Vitello intestinal duct
c) Left umbilical vein
d) Obliterated umbilical artery
ГОУ ВПО «Смоленская государственная медицинская академия
Федерального агентства по здравоохранению и социальному развитию»
МЕТОДИЧЕСКИЕ УКАЗАНИЯ ДЛЯ СТУДЕНТОВ
ПО ДИСЦИПЛИНЕ хирургические болезни
ACUTE CHOLECYSTITIS
Составитель доц. А.Ю.Некрасов
Методические указания утверждены на методическом совещании кафедры госпитальной хирургии (протокол № 2 от 6 октября 2008 г.)
Зав. кафедрой______________(проф. С.А.Касумьян)
2008 г.
QUESTIONS FOR HOMEWORK:
Anatomy of extrahepatic biliary tract
Biliary physiology
Acute cholecystisis
Management of acute cholecystisis
Complications of acute cholecystisis
Treatment acute cholecystisis
Postcholecystecomy syndrome
ANATOMY OF EXTRAHEPATIC BILIARY TRACT
The extrahepatic biliary tract consists of the bifurcation of the left and right hepatic ducts, the common hepatic duct and common bile duct, and the cystic duct and gallbladder (Fig. 1). The left hepatic duct is formed by the ducts draining segments II, III, and IV of the liver, courses horizontally along the base of segment IV;
and has an extrahepatic length of 2 cm or more. The right hepatic duct is formed by the right posterior (segments VI and VII) and the right anterior (segments V and VIII) hepatic ducts and has a short extrahepatic length. The hepatic duct bifurcation is usually extrahepatic and anterior to the portal vein bifurcation. The common hepatic duct lies anteriorly in the hepatoduodenal ligament and joins the cystic duct to form the common bile duct. The common bile duct extends from the cystic duct-common hepatic duct junction inferiorly to the papilla of Vater, where it empties into the duodenum. The common bile duct varies in length from 5 to 9 cm, depending on its junction with the cystic duct and is divided into three segments: supraduodenal, retroduodenal, and intrapancreatic. The distal common bile duct and the pancreatic duct may join outside the duodenal wall to form a long common channel within the duodenal wall to form a short common channel, or they may enter the duodenum through two distinct ostia.
The gallbladder is a pear-shaped reservoir in continuity with the common hepatic and common bile ducts via the cystic duct. The gallbladder lies on the inferior surface of the liver partially enveloped in a layer of peritoneum. The gallbladder is anatomically divided into the fundus, body, infundibulum, and neck, which empties into the cystic duct. Both the gallbladder neck and the cystic duct contain spirally oriented mucosal folds known as the valves of Heister. The cystic duct varies in length from 1 to 4 cm, usually joining the common hepatic duct at an acute angle.
The gallbladder is supplied by the cystic artery, which most commonly is a single branch of the right hepatic artery The cystic artery may also originate from the left hepatic, common hepatic, gastroduodenal, or superior mesenteric artery. The cystic artery is usually located parallel and medial to the cystic duct, but its course varies with its origin. The cystic artery divides into superficial and deep branches before entering the gallbladder.
The blood supply to the extrahepatic biliary tree originates distally from the gastroduodenal, retroduodenal, and posterior superior pancreatoduodenal arteries and proximally from the right hepatic and cystic arteries.
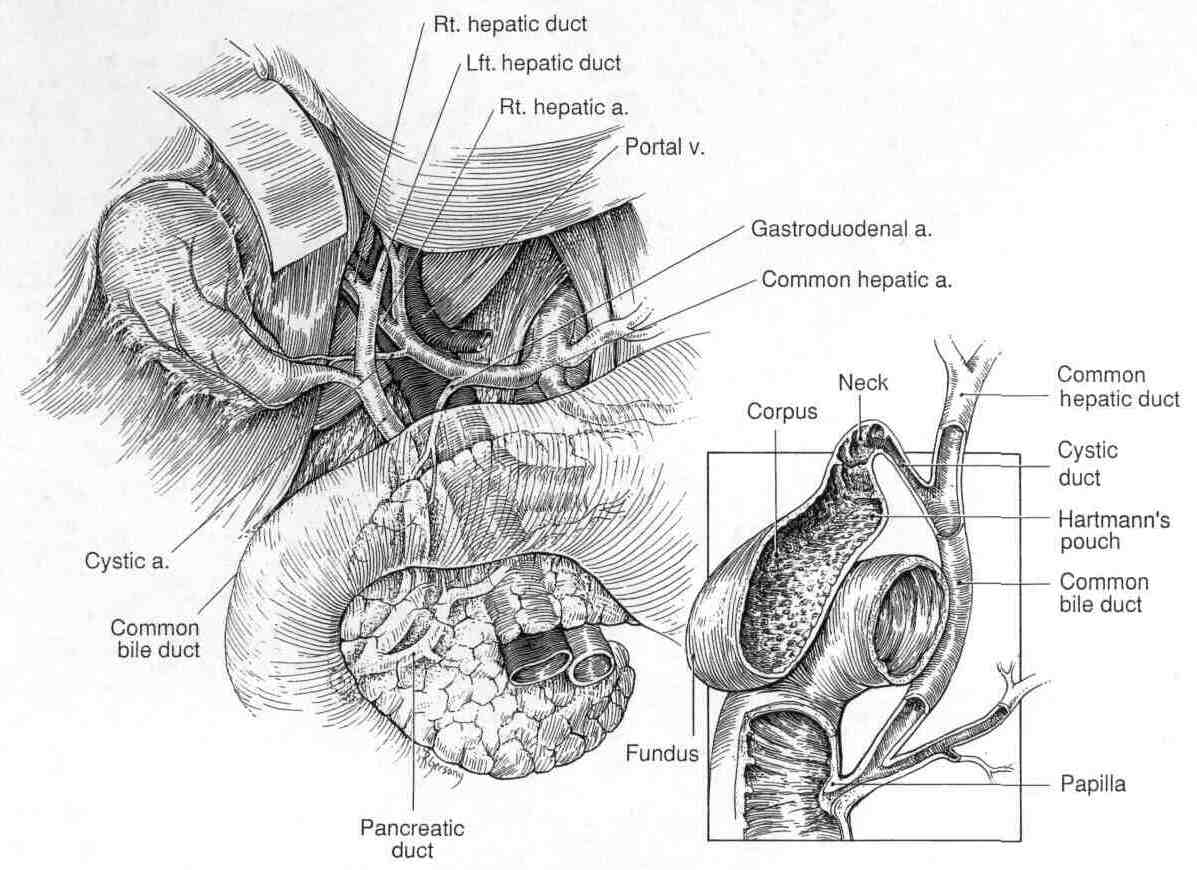
Figure 1. Anatomy of the biliary system and its relationship to surrounding structures.
BILIARY PHYSIOLOGY
The bile ducts, gallbladder, and sphincter of Oddi act in concert to modify, store, and regulate the flow of bile. During its passage through the bile ductules and the hepatic duct, canalicular bile is modified by the absorption and secretion of electrolytes and water. The gastrointestinal hormone secretin increases bile flow primarily by increasing the active secretion of chloride-rich fluid by the bile ducts and ductules. Bile ductular secretion is also stimulated by other hormones, such as cholecystokinin and gastrin. The bile duct epithelium is also capable of water and electrolyte absorption, which may be of primary importance in the storage of bile during fasting in patients who have previously undergone cholecystectomy. The main function of the gallbladder is to concentrate and store hepatic bile during the fasting state and deliver bile into the duodenum in response to a meal. The usual capacity of the human gallbladder is only about 40 to 50 ml. Only a small fraction of the 600 ml of bile produced each day would be stored were it not for its remarkable absorptive capacity. The gallbladder mucosa has the greatest absorptive capacity per unit area of any structure in the body. Bile is usually concentrated 5- to 10-fold by the absorption of water and electrolytes, leading to a marked change in bile composition.
ACUTE CALCULOUS CHOLECYSTISIS
Pathophysiology - in 90 to 95% of cases, acute cholecystitis is related to gallstones. Obstruction of the cystic duct by a gallstone leads to biliary colic and is also the first event in acute cholecystitis. If the cystic duct remains obstructed, the gallbladder distends, and the gallbladder wall becomes inflamed and edematous. In the most severe cases (5 to 10%), this process can lead to ischemia and necrosis of the gallbladder wall. More frequently, the gallstone is dislodged, and the inflammation gradually resolves. Initially, acute cholecystitis is an inflammatory process. Approximately 50% of patients with uncomplicated acute cholecystitis have positive bile cultures at the time of cholecystectomy. In the most severe cases, generalized sepsis may be present.
Clinical presentation - right upper quadrant abdominal pain is the most common complaint in patients with acute cholecystitis. The pain may be similar to that in previous episodes of biliary colic, but the pain of acute cholecystitis persists for longer than an uncomplicated episode of biliary colic (days versus several hours). Other common symptoms include nausea, vomiting, and fever. On physical examination, focal tenderness and guarding are usually present inferior to the right costal margin, distinguishing the episode from simple biliary colic. A mass may be present in the right upper quadrant (gallbladder with adherent omentum), and a Murphy sign (inspiratory arrest with deep palpation in the right upper quadrant) may also be elicited. A mild leukocytosis is usually present (12,000 to 14,000 cells/mm3). In addition, mild elevations in serum bilirubin (<4 mg/ dl), alkaline phosphatase, transaminases, and amylase may be present.
Diagnosis - ultrasound is the most useful radiologic examination in the patient with suspected cholecystitis (Fig. 2). First, in the patient without known gallstones, ultrasound is a sensitive test for establishing the presence or absence of gallstones. Additional findings suggestive of acute cholecystitis include thickening of the gallbladder wall (>4 mm) and pericholecystic fluid. Focal tenderness directly over the gallbladder (sonographic Murphy sign) is also suggestive of acute cholecystitis. Ultrasound has a sensitivity and a specificity of 85% and 95%, respectively, for diagnosing acute cholecystitis.
Radionuclide scanning is used less frequently for the diagnosis of acute cholecystitis but may provide additional information in the atypical case. Nonfilling of the gallbladder with the radiotracer 99Tc-hepato-iminodiacetic acid (HIDA) indicates an obstructed cystic duct and, in certain clinical settings, is highly sensitive and specific for acute cholecystitis.

Figure 2.
A. Gallbladder ultrasound in patient with acute cholecystitis demonstrates gallbladder wall thickening (4.2 mm) and pericholecystic fluid.
B. Abdominal CT scan in same patient shows distended, thick-walled gallbladder with pericholecystic fluid.
MANAGEMENT OF ACUTE CALCULOUS CHOLECYSTISIS
Antibiotic selection - Bile in the gallbladder or bile ducts in the absence of gallstones or any other biliary tract desease is normally sterile. But in the presence of gallstones or biliary obstruction, the prevalence of bactibilia increases. The presence of positive bile cultures is influenced by several factors, including the severity or the type of biliary disease and the patient’s age.
Gram-negative aerobes are the organisms most frequently isolated from bile in patients with symptomatic gallstones, acute cholecystitis, or cholangitis. Escherichia coli and Klebsiella species are the most common gram-negative bacteria isolated. However, the more resistant organisms Pseudomonas and Enterobacter are being seen with increased frequency, particularly in patients with malignant biliary obstruction, who may have been treated with antibiotics previously for a biliary tract infection. Other common isolates include the gram-positive aerobes, enterococcus and Streptococcus viridans. Anaerobes, such as Bacteroides species and Clostridium, continue to play a small but significant role in biliary infections. The prevalence of anaerobic bacteria is 10 to 13% in patients with acute cholecystitis or cholangitis. Candida species are also being increasingly recognized as a significant biliary pathogen, particularly in critically ill patients. In most patients with symptomatic cholelithiasis, acute cholecystitis, or common bile duct stones in the absence of cholangitis, a single organism is isolated in bile cultures. Polymicrobial infections are more common (32 to 37%) in patients with cholangitis. In analyzing response to therapy, the isolation of Candida, panresistant bacteria, and more than two bacteria are associated with treatment failures.
The source of bacteria in patients with biliary tract infections is controversial. Most evidence favors an ascending route via the duodenum as the main source of biliary bacteria. The bacterial flora in the small intestine is similar to that detected in the biliary tract. In addition, in most patients, gallbladder and common bile duct cultures yield a similar result. Furthermore, the prevalence of bactibilia is highest in the elderly, in whom biliary motility and clearance have decreased.
This is why antibiotics should be used prophylactically in all patients undergoing elective biliary tract surgery or other biliary tract manipulations, such as endoscopic and percutaneous cholangiography. The risk of postoperative infectious complications corresponds to the presence of bactibilia, which occurs in 11 to 30% of patients with gallstones, but is difficult to determine before surgery. In patients undergoing laparoscopic cholecystectomy for chronic cholecystitis, the risks associated with a single dose of the first-generation cephalosporin cefazolin, are minimal, and this agent provides good coverage against the gram-negative aerobes commonly isolated from bile and skin flora.
Therapeutic antibiotics are used in patients with acute cholecystitis and acute cholangitis. In both diseases, gram-negative aerobes play a major role and are well covered by the second- or third-generation cephalosporins, aminoglycosides, ureidopenicillins, carbapenems, and fluoroquinolones. Ureidopenicillins, such as piperacillin, offer the advantages of gram-positive coverage, including against the enterococci, and anaerobic coverage. When combined with a beta-lactamase inhibitor, such as tazobactam, piperacillin offers extended and improved coverage against organisms with acquired resistance. Most fluoroquinolones, such as ciprofloxacin, do not cover the anaerobes and should be used in combination with an agent that provides anaerobic coverage (e.g., metronidazole).
Once the diagnosis of acute cholecystitis is made, the patient's oral intake should be limited and intravenous antibiotics should be given. An appropriate antibiotic for the common biliary tract pathogens isolated from the bile in patients with acute cholecystitis should be selected. Parenteral analgesia should also be administered. Unfortunately, narcotics increase biliary pressure, whereas nonsteroidal analgesics, which inhibit prostaglandin synthesis, reduce gallbladder mucin production and, therefore, reduce pressure and pain.
Open cholecystectomy has been the standard treatment for acute cholecystitis for many years. At the time laparoscopic cholecystectomy was introduced, acute cholecystitis was a relative contraindication to the use of the laparoscopic technique. However, with increased experience, the results of laparoscopic cholecystectomy are as good as or better than open cholecystectomy. The morbidity rate, hospital stay, and time to return to work have all been lower in patients undergoing laparoscopic cholecystectomy than in those undergoing open cholecystectomy in prospective, randomized trials.
The timing of cholecystectomy for acute cholecystitis has been studied for several decades and has been further evaluated by use of laparoscopic cholecystectomy as the primary therapy. Several retrospective series have demonstrated advantages to proceeding with laparoscopic cholecystectomy soon after the diagnosis of acute cholecystitis is made. Patients operated on early in the course of their illness (within several days of the onset of symptoms) were more likely to have the procedure completed laparoscopically than were those with symptoms for greater than 5 days.
COMPLICATIONS OF ACUTE CALCULOUS CHOLECYSTISIS
Acute cholecystitis may progress to empyema of the gallbladder, emphysematous cholecystitis, or perforation of the gallbladder, despite antibiotic therapy. In each case, emergency cholecystectomy is warranted, if the patient can withstand an anesthetic.
Empyema occurs with bacterial proliferation in an obstructed gallbladder and results in a pus-filled organ. Patients with empyema of the gallbladder may be toxic and have more marked fevers and leukocytosis. Laparoscopic cholecystectomy may be attempted, but the conversion rate is high.
Emphysematous cholecystitis develops more commonly in males and in patients with diabetes mellitus. Severe right upper quadrant pain and generalized sepsis are frequently present. Abdominal films or CT scans may demonstrate air within the gallbladder wall or lumen. Prompt antibiotic therapy to cover the common biliary pathogens (e.g., E. coli Enterococcus, Klebsiella) and Clostridia species and emergency cholecystectomy are appropriate treatments.
Perforation of the gallbladder occurs in up to 10% of cases of acute cholecystitis. Perforation is a sequela of ischemia and gangrene of the gallbladder wall and occurs most commonly in the gallbladder fundus. The perforation is most frequently (50% of cases) contained within the subhepatic space by the omentum, duodenum, liver, and hepatic flexure of the colon, and a localized abscess forms. Less commonly, the gallbladder perforates into an adjacent viscus (duodenum or colon), resulting in a cholecystoenteric fistula. Rarely, the gallbladder perforates freely into the peritoneal cavity, leading to generalized peritonitis. With gallbladder perforation, the abdominal tenderness, fever, and white blood cell count are more pronounced or higher than in uncomplicated acute cholecystitis. Localized right upper quadrant pain and tenderness, which becomes diffuse and generalized, should raise the suspicion of free gallbladder perforation. Intravenous fluids, antibiotics, and emergency cholecystectomy is the treatment of choice in patients with gallbladder perforation.
In most patients, cholecystectomy can be performed and is the best treatment for complicated acute cholecystitis. Occasionally, the inflammatory process obscures the structures in the triangle of Calot, precluding safe dissection or ligation of the cystic duct. In these patients, partial cholecystectomy, cauterization of the remaining gallbladder mucosa, and drainage avoid injury to the common bile duct. In patients considered too unstable to undergo laparotomy because of concurrent medical comorbidities, percutaneous transhepatic cholecystostomy can drain the gallbladder. Success rates approaching 90% have been reported with percutaneous cholecystostomy in the treatment of critically ill patients believed to have acute cholecystitis. However, this procedure leaves in the gallbladder, which may be partially gangrenous and a source of ongoing sepsis.
ACUTE ACALCULOUS CHOLECYSTISIS
Acute acalculous cholecystitis accounts for 5 to 10% of all cases of acute cholecystitis and is the diagnosis in approximately 1 to 2% of patients undergoing cholecystectomy. The disease often has a more fulminant course than acute calculous cholecystitis and frequently progresses to gangrene, empyema, or perforation. Acute acalculous cholecystitis usually occurs in the critically ill patient after trauma, burns, long-term parenteral nutrition, major nonbiliary operations, and cardiopulmonary bypass. The cause of acute acalculous cholecystitis remains unclear, although gallbladder stasis and ischemia have been most often implicated as causative factors. Stasis is common in critically ill patients who are not being fed enterally and may lead to colonization of the gallbladder with bacteria. Visceral ischemia is also a common denominator in patients with acute acalculous cholecystitis and may explain the high incidence of gallbladder gangrene. Microangiography in patients with acute acalculous cholecystitis has demonstrated a decrease in arteriolar and capillary filling, in contrast to the dilatation of these vessels observed in acute calculous cholecystitis.
The symptoms and signs of acute acalculous cholecystitis are similar to acute calculous cholecystitis, with right upper quadrant pain and tenderness, fever, and leukocytosis most frequently present. However, these findings are often masked by other conditions in the critically ill patient. CT scan and ultrasound findings are similar to those in calculous cholecystitis and include gallbladder wall thickening and pericholecystic fluid in the absence of gallstones. Cholescintigraphy demonstrates absent gallbladder filling in acute acalculous cholecystitis. However, the false-positive rate (absent gallbladder filling without acute acalculous cholecystitis) may be as high as 40%. Morphine cholescintigraphy has improved the accuracy of this study in the critically ill patient.
Emergency cholecystectomy is the appropriate treatment once the diagnosis is established or the suspicion is high. The incidence of gangrene, perforation, and empyema exceeds 50%, and therefore, open cholecystectomy is usually required in this setting. The mortality rate for acute acalculous cholecystitis in series remains high (40%), in large part because of the concomitant illnesses in patients who develop this disease.
CHOLECYSTECOMY: INDICATIONS AND TECHNIQUE
Cholecystectomy is one of the most common gastrointestinal operations. Since the introduction of laparoscopic cholecystectomy, the number of cholecystectomies has increased dramatically. Most conditions initially believed to be relative contraindications early in the laparoscopic experience are no longer believed to mandate an open cholecystectomy. Uncontrolled coagulopathy is one of the few current contraindications to laparoscopic cholecystectomy. In addition, patients with severe chronic obstructive pulmonary disease or congestive heart failure may not tolerate the pneumoperitoneum required for performing laparoscopic surgery. Currently, the major contraindication to completing a laparoscopic cholecystectomy is an inability to clearly identify all of the anatomic structures. A liberal policy of converting to an open operation when important anatomic structures cannot be clearly defined represents good surgical judgment rather than a complication.
The technical difficulty of laparoscopic cholecystectomy is increased in several clinical settings. Laparoscopic cholecystectomy can be performed safely in acute cholecystitis, albeit with a higher conversion rate and operative time than in the elective setting. Morbid obesity, once believed to be a relative contraindication to the laparoscopic approach, is not associated with a higher conversion rate. Longer trocars and instruments and increased intra-abdominal pressure may be helpful in these patients. Prior upper abdominal surgery may increase the difficulty of, or preclude the use of, laparoscopic cholecystectomy. However, placement of a Hasson cannula often reveals few adhesions or adhesions that can be dissected laparoscopically, permitting completion of a laparoscopic cholecystectomy. Laparoscopic cholecystectomy has also been completed safely in patients with cirrhosis, although difficulty retracting the firm liver and increased bleeding from collaterals have been noted.
LAPAROSCOPIC CHOLECYSTECOMY
Patients undergoing laparoscopic cholecystectomy are prepared and draped in a fashion similar to open cholecystectomy. Conversion to an open operation should be discussed with the patient, included in the operative consent.
Laparoscopic surgery requires a space for visualization and instrument manipulation, and this space is usually created by establishing a pneumoperitoneum with carbon dioxide. Both open and closed methods have been used to establish a pneumoperitoneum. With the open technique, a small incision is made above the umbilicus into the peritoneal cavity. A special blunt-tipped cannula (Hasson) with a gas tight sleeve is inserted into the peritoneal cavity and anchored to the fascia. This technique is often used after previous abdominal surgery and should avoid infrequent, but potentially life-threatening, trocar injuries. In the closed technique a special hollow insufflation needle (Veress) with a retractable cutting sheath is inserted into the peritoneal cavity through a supraumbilical incision and used for insufflation.
Once an adequate pneumoperitoneum has been established, a 10-mm trocar is inserted through the supraumbilical incision. The laparoscope with attached video camera is then inserted through the umbilical port and an examination of the peritoneal cavity is performed. With either the open or the closed techniques, additional trocars are inserted under direct vision. Most surgeons use a second 10-mm trocar placed subxiphoid and two additional 5-mm trocars positioned subcostally in the right upper quadrant in the midclavicular and anterior axillary lines.
This maneuver may require bluntly taking down any adhesions between the omentum or duodenum and the gallbladder. The junction of the gallbladder and cystic duct is identified by stripping the peritoneum off the gallbladder neck and removing any tissue surrounding the gallbladder neck and the proximal cystic duct. Once the cystic duct is identified, an intraoperative cholangiogram may be performed by placing a hemoclip proximally on the cystic duct, incising the anterior surface of the duct, and passing a cholangiogram catheter into the cystic duct. Once the cholangiogram is completed, two clips are placed distally on the cystic duct, and it is divided. A large cystic duct may require placement of a pretied loop ligature to provide a secure closure.
The next step is the identification and division of the cystic artery. The artery is usually encountered running parallel to and behind the cystic duct. Once the artery is identified and isolated, clips are placed proximally and distally on the artery, and it is divided. Once the artery and any branches are controlled, the gallbladder is dissected out of the gallbladder fossa by use of either a hook or spatula cautery. The peritoneum overlying the gallbladder is placed on tension by use of the two grasping forceps, and the peritoneum and adventitia between the gallbladder and liver are divided with the cautery. Just before the gallbladder is removed from the liver, the operative field is carefully searched for hemostasis, and adequate placement of the cystic duct and artery clips is confirmed. The gallbladder is then dissected off the liver and is usually removed through the umbilical port. The fascial defect and skin incision may need to be enlarged to remove the gallbladder and contained gallstones. If the gallbladder has been entered during the dissection or if it is acutely inflamed or gangrenous, the gallbladder may be placed in a plastic specimen retrieval bag before it is removed from the peritoneal cavity.
OPEN CHOLECYSTECOMY
Open cholecystectomy can be performed through either an upper midline or a right subcostal (Kocher) incision. Identification and division of the cystic duct and artery initially limit bleeding from the gallbladder for the remainder of the dissection. With lateral traction on the gallbladder neck, the peritoneum overlying the triangle of Calot is incised, and the cystic duct is identified and ligated. Cholangiography is performed at this time if indicated. The cystic duct is then ligated both proximally and distally and divided. Similarly, the cystic artery is ligated and divided after it is carefully traced onto the gallbladder.
If the anatomy cannot be clearly identified, the gallbladder should be dissected from the fundus downward toward the gallbladder neck, making the ductal and vascular anatomy easier to identify. The gallbladder is dissected out of the gallbladder bed by incising the overlying peritoneum with cautery. At this point, cystic duct cholangiography is performed. Rarely, a small duct entering the gallbladder from the liver is encountered and should be ligated. A closed suction drain is placed if there is concern about the security of the cystic duct closure (e.g., as in gangrenous cholecystitis).
POSTCHOLECYSTECOMY SYNDROME
Abdominal pain or other symptoms originally attributed to the gallbladder may persist or recur months or years after cholecystectomy. Recurrence of pain or other symptoms after cholecystectomy has been reported in as many as 20% of patients. However, with improvements in biliary imaging over the past decade, the incidence of "postcholecystectomy syndrome" has certainly decreased. Several common causes of postcholecystectomy pain are listed in Table 1. Episodic right upper quadrant pain associated with jaundice and chills occurring shortly after cholecystectomy is most commonly associated with a retained common bile duct stone or bile duct injury or leak. Acute epigastric pain not associated with jaundice may be due to unrecognized pancreatitis, peptic ulcer disease, or even irritable bowel syndrome. Formerly, a long cystic duct stump was believed to be a potential source of symptoms after cholecystectomy. However, with the laparoscopic technique, the cystic duct is left long by design to minimize the risk of bile duct injuries, and no increased risk of biliary symptoms has been observed. Finally, a small group of patients have persistent biliary-type pain after cholecystectomy as a result of abnormalities in the sphincter of Oddi.
TABLE 1. Causes of postcholecyctecomy pain
BILIARY |
NONBILIARY |
Choledocholithiasis Bile duct stricture/injury Sphincter of Oddi dysfunction Stenosing papllitis |
Pancreatitis Irritable bowel syndrome Peptic ulcer disease Gastroesophageal reflux disease Liver disease Wound neuroma |
TESTS
1. Commonest type of gall stone is -
a) Cholesterol stone
b) Pigment
c) Mixed
d) All are equally common
2. Percentage of gall stones which are radio opaque-
a) 10%
b) 20 %
c) 30 %
d) 50 %
e) 80 %
3. Features of emphysematous cholecystitis to include all except -
a) Elderly male patient
b) Diabetic
c) Cl. welchi is the infecting organism
d) Gas in the gall bladder
e) Good prognosis
4. A gall stone gets impacted most commonly in which part of common bile duct -
a) Supraduodenal
b) Retroduodenal
c) Ampulla of vater
d) Common hepatic duct
5. Common bile duct stones will manifest all except -
a) Distended gall bladder
b) Jaundice
c) Itching
d) Clay coloured stools
6. In cholangitis, the organism mostly responsible is -
a) E. coli
b) Streptococcus
c) E. Histolytica
d) Clostridium
7. Best investigative modality for gall bladder -
a) OCG
b) PTC
c) Ultrasound
d) Intravenous cholangiogram
8. Which does not contribute to Enterobiliary Fistula -
a) Duodenal ulcer
b) Gall stones
c) Gastric ulcer
d) Carcinoma gall bladder
9. Chemical mediator of acute cholecystitis is -
a) Lysolecithin b) Bilirubin
c) Cholic acid
d) Cholesterol
10. Commonest cause of hemobilia is -
a) Gall stones
b) Trauma
c) Cholangitis
d) Hepatoma
11. Investigation of choice in gall bladder stone is -
a) USG
b) X-ray abdomen
c) OCG
d) Intravenous cholangiogram
12. Laparoscopic cholecystectomy is best avoided in patient with -
a) Hypertension
b) Diabetes
c) Obesity
d) COPD (chronic obstructive pulmonary disease)
ГОУ ВПО «Смоленская государственная медицинская академия
Федерального агентства по здравоохранению и социальному развитию»
МЕТОДИЧЕСКИЕ УКАЗАНИЯ ДЛЯ СТУДЕНТОВ
ПО ДИСЦИПЛИНЕ хирургические болезни
ACUTE PANCREATITIS. CHRONIC PANCREATITIS, CYSTES OF THE PANCREAS
Составитель асс. А.А.Безалтынных
Методические указания утверждены на методическом совещании кафедры госпитальной хирургии (протокол № 2 от 6 октября 2008 г.)
Зав. кафедрой______________(проф. С.А.Касумьян)
2008 г.
QUESTIONS FOR HOMEWORK:
1. Anatomy
2. Classification
3. Pathophysiology
4. Clinical symptoms.
5. Diagnostics.
6. Differential diagnosis.
7. Complications
8. Treatment.
9. Postoperative complications.
INTRODUCTION
Controversy still surrounds the classification of acute and chronic pancreatitis. The most recent classifications define acute pancreatitis as a spectrum of inflammatory lesions in the pancreas and peripancreatic tissue with variable degrees of oedema, necrosis, and haemorrhage. Exocrine and endocrine function may be impaired to a variable extent and for a variable length of time. If the cause can be rectified and complications such as pseudocysts dealt with, then a clinical return to functional and morphological normality is usual. Acute pancreatitis rarely progresses to chronic pancreatitis.
Chronic pancreatitis is almost always associated with recurring or persisting pain. Chronic inflammation causes fibrosis with destruction of exocrine parenchyma leading to malabsorption and steatorrhoea. In the later stages, diabetes mellitus may follow destruction of the endocrine parenchyma.
INCIDENCE AND PREVALENCE
Chronic pancreatitis is relatively rare. Autopsy estimates of prevalence range from 0.03 to 0.4 percent, but care has to be taken in the interpretation of postmortem findings. Pancreatic calculi are not uncommon after the age of 70 and are often associated with atrophy and fibrosis. However, these changes may be the result of squamous metaplasia of the ductal epithelium, are not associated with alcohol abuse, and do not produce the clinical picture of chronic pancreatitis.
In Europe and North America the incidence of chronic pancreatitis appears to be increasing, although this may reflect increased awareness and improved diagnosis. The prospective Copenhagen Pancreatitis Study found an annual incidence of 8.2 cases per 100000 and a prevalence of 27.4 per 100000. Extrapolation from these figures predicts a mean life expectancy of only 3.3 years from diagnosis, an estimate which is clearly too low, and it may be that the prevalence figure is an underestimate. In general, countries with a low alcohol intake have a low incidence of chronic pancreatitis, although there are many discrepancies. For example, Switzerland has a higher per capita alcohol consumption than Denmark and Sweden but a much lower incidence of chronic pancreatitis.
AETIOLOGY, PATHOGENESIS, AND PATHOLOGY
Two forms of chronic pancreatitis are generally recognized a common calcificform and a less common obstructive form. Some accept the existence of a third inflammatory form which can only be identified on histological examination and will not be considered further.
Chronic calcific pancreatitis
This type of pancreatitis is found in association with alcohol abuse (which now accounts for some 70 per cent of all cases in Western centres), hypercalcaemia, and malnutrition, and in hereditary and idiopathic chronic pancreatitis. The disease has a patchy lobular distribution, at least in its early stages, and inflammation leads to fibrosis, destruction of parenchyma, and eventual atrophy. Dilatation of the pancreatic duct system is often prominent. A common pathogenesis has been proposed in which the ductal system becomes occluded by protein precipitates or plugs which subsequently calcify. It is postulated that overstimulation of the acinar cells deranges intracellular transport of secretory proteins, resulting in an abnormal admixture of digestive enzymes and lysosomal hydrolases and/or storage of zymogens in acid compartments. Secretion of the iron binding protein, lactoferrin, is also increased; this molecule is known to associate strongly with acidic molecules to form complexes, a property that might facilitate formation of protein precipitates. In addition to secretory protein, pancreatic juice also contains a so-called pancreatic stone protein which can prevent nucleation and growth of calcium carbonate crystals.
Alcohol raises the viscosity of pancreatic juice by increasing secretory protein output and lactoferrin concentration, but it may also decrease biosynthesis of pancreatic stone protein (or increase its denaturation). Thus, the stage is set for the formation of protein plugs in the lumen of the acini and small ducts, and for their subsequent calcification. The calculi so formed consist of calcium carbonate, usually in the form of calcite (which constitutes approximately 95 per cent of their weight) and eosinophilic protein fibrils, which are thought to be a degraded form of pancreatic stone protein.
Although alcohol is heavily implicated in protein plug formation, less than 10 per cent of alcoholics develop chronic pancreatitis, and other factors must be involved. In addition, many patients with chronic pancreatitis have not abused alcohol and some are teetotal. Evidence suggesting that alcohol is more likely to cause chronic pancreatitis in individuals consuming a diet high in fat and protein is now contested: inadequate intake of antioxidant vitamins and trace elements may be more important. Induction of the detoxifying P450-I cytochromes occurs in the majority of patients with chronic pancreatitis regardless of cause. Such induction increases the production of oxygen free radicals and reactive toxic intermediates derived from ingested drugs and chemicals. If antioxidant intake is insufficient, failure of normal protective mechanisms could allow organelles within pancreatic acinar cells to become damaged. The trace elements zinc, copper, and manganese are required for activity of the free radical scavenger superoxide dismutase, while selenium is an essential component of glutathione peroxidase, an enzyme which controls endogenous peroxidase formation from unsaturated fatty acids in cell membranes. Alcoholics who smoke are at a higher risk of developing chronic pancreatitis than those who do not: cigarette smoking may increase free radical formation while at the same time diminishing antioxidant levels and the ability to scavenge free radicals.
A tropical form of chronic calcific pancreatitis is well recognized in underdeveloped areas of Africa and India, with a particularly high incidence in the Indian state of Kerala. Tropical pancreatitis characteristically presents with recurrent abdominal pain in childhood, development of diabetes at around puberty and death in early adulthood. The pancreatitis is associated with marked calcification and was once thought to result from the protein calorie malnutrition of kwashiorkor. However, it now seems more likely that pancreatic pain and insufficiency cause malnutrition rather than result from it. Dietary toxins, such as the cyanogenic glycosides found in cassava, a staple consumed in many areas affected by tropical pancreatitis, have also been implicated.
The incidence of chronic pancreatitis in patients with hyperparathyroidism has fallen from 5 to 10 per cent to around 1 to 2 per cent, probably as a result of earlier diagnosis and treatment of the parathyroid disorder. Hypercalcaemia stimulates the acinar cell and may also increase the concentration of calcium in pancreatic juice, thus favouring calculi formation.
Hereditary pancreatitis is a rare autosomal dominant disorder with incomplete penetrance. Pancreatitis is of early onset, calcification is marked, and there may be hyperlipidaemia and disorders of amino acid metabolism. The condition may increase the risk of pancreatic cancer, a risk reported to be as high as 25 per cent in some series. As in idiopathic pancreatitis (which is now thought to account for 10 to 30 per cent of cases of chronic pancreatitis) the mechanisms involved in the production of pancreatic inflammation are obscure but reduction in pancreatic stone protein levels may be involved.
Obstructive chronic pancreatitis
Obstruction of the duct due to papillary stenosis, scarring from acute pancreatitis or trauma, cysts or pseudocysts, or neoplasia may produce generalized diffuse inflammation and duct dilatation in the pancreas upstream of the point of blockage. Recurrent attacks of acute inflammation frequently precede chronic inflammation and more persistent pain. Characteristically, these acute attacks are less severe clinically than those which often develop in acute pancreatitis due to gallstones, and life threatening progression to necrosis and abscess formation is exceptional.
It is debatable whether pancreas divisum is a cause of obstructive pancreatitis. In pancreas divisum there is failure of fusion of the embryological dorsal and ventral pancreas so that most of the exocrine secretion has to pass through the smaller accessory papilla to gain the duodenum. The widespread use of endoscopic retrograde cholangiopancreatography (ERCP) indicates that pancreas divisum may be present in up to 10 per cent of the population, calling into question its role in pancreatitis. Nevertheless, pancreas divisum may be present in some patients with chronic pancreatitis and it may be such patients have superimposed papillary stenosis.
In contrast to chronic calcific pancreatitis, there may be considerable clinical and morphological improvement in obstructive pancreatitis once the obstructing lesion has been dealt with.
CLINICAL FEATURES
Pain
Only about 5 per cent of patients with chronic pancreatitis do not experience pain. In the remainder pain is usually the cardinal symptom, and it is by far the most common indication for surgery. The pain is recurrent or persistent, often incapacitating, and frequently requires administration of opiates for relief. It is usually experienced in the upper abdomen with radiation through to the back and is poorly localized, but may be lateralized to one or other hypochondrium and lower chest. The back pain can be particularly distressing. Some patients gain relief by leaning forwards while sitting, while some resort to kneeling on all fours. Lying flat in bed can be extremely painful and many patients sleep with the most painful side uppermost. Pain is often, but by no means invariably, exacerbated by food and alcohol intake and fatty foods may be particularly troublesome. Patients frequently apply heat to painful areas and permanent skin mottling (erythema ab igne) may result.
Attacks of acute inflammation may cause exacerbations of pain lasting for some days in the early stages of chronic pancreatitis. Although such exacerbations seldom produce a severe clinical illness, each attack should still be managed from the outset as a potentially life-threatening illness. With progression of chronic pancreatitis, acute exacerbations become less frequent and less severe, and serum amylase and lipase elevations become less marked as the gland loses its secretory capacity.
Obstruction of the pancreatic duct may cause attacks of pain which begin after eating and last for some hours. Pressure in the pancreatic duct is increased in most patients with chronic pancreatitis and it is tempting to assume that eating triggers exocrine secretion, increasing duct pressure and causing duct dilatation and pain. Pancreatic interstitial pressure is also raised and in general, operations designed to improve drainage reduce ductal and interstitial pressure. However, some patients with duct dilatation do not appear to suffer much pain, while others have pain in the absence of dilatation.
Pancreatic cysts and pseudocysts are common in patients with chronic pancreatitis and may cause pain as a consequence of the pressure within the fluid collection. While drainage often relieves pain, there is a variable relationship between pain, the presence of pseudocysts, and the magnitude of intracyst pressure.
Attention has centred recently on morphological changes in the nerves supplying the pancreas and their role in pain production. The mean diameter of the nerves is increased while the mean area served by each nerve is reduced. Oedema in nerve bundles is common and the perineurial sheath appears to serve as a less effective barrier between the surrounding connective tissue and its contents. Perineurial inflammation may be present, although its extent does not correlate with pain severity. Immunohistochemical studies suggest that there are increased amounts of neurotransmitters, such as substance P, in the nerve sheaths. Eosinophilic infiltration of the perineurial space has also been reported, particularly in those who have ingested alcohol recently, and the release of neurotoxins or pain mediators from eosinophils or mast cells has been suggested as a cause of pain.
Extrapancreatic causes of pain in chronic pancreatitis include stenosis or compression of the duodenum and common bile duct, and splenic vein thrombosis with splenic infarction. Duodenal and bile duct narrowing may result from fibrosis caused by long-standing pancreatic inflammation, from oedema in an acute exacerbation of pancreatitis, or from a pseudocyst. It is often difficult to determine the contribution of such extrapancreatic lesions to pain production but in general, pain is more often due to pancreatic causes. Duodenal ulceration is another potential cause of pain in chronic pancreatitis, the increased incidence of ulceration reflecting gastric hypersecretion and the diminished capacity of the pancreas to secrete bicarbonate and so neutralize acid in the duodenal bulb.
It is clear that there is no single cause of the pain of chronic pancreatitis and that there is great variation in its severity between individuals. Operations to relieve pain frequently have to be undertaken earlier in patients with alcohol-induced disease. Gross impairment of function with loss of secretory capacity and development of marked pancreatic calcification may be associated with increasing freedom from pain, but in many patients, an expectant approach is prevented by continuing severe pain and fears of opiate addiction.
Exocrine insufficiency
Development of steatorrhoea indicates that pancreatic exocrine secretory capacity has fallen to less than 10 per cent of normal. The stools are bulky, pale, difficult to flush and have a particularly offensive and pervasive smell which causes considerable embarrassment. Inadvertent passage of oily droplets with staining of underwear can also be a problem. Watery diarrhoea is unusual because fat is not hydrolysed to fatty acids until it has been exposed to bacterial lipase in the colon; thus fatty acids are not present in the small intestine to trigger a secretory diarrhoea. If watery diarrhoea does occur in chronic pancreatitis it may reflect failure of pancreatic bicarbonate secretion with lowering of intraduodenal pH, precipitation of bile salts, and failure to solubilize fatty acids for absorption.
Excessive loss of dietary protein from failure to secrete proteases is seldom as marked as steatorrhoea, but contributes to the weight loss and muscle wasting seen in patients with severe chronic pancreatitis.
Endocrine insufficiency
Failure of pancreatic endocrine function does not necessarily parallel the decline in exocrine function and occurs at a relatively late stage. When diabetes mellitus does develop it seldom responds to oral hypoglycaemic agents and exogenous insulin has to be prescribed. There is a real danger of insulin sensitivity as glucagon secretion is also reduced, with a resultant lowering of the capacity for gluconeogenesis. Insulin therapy must be monitored carefully and the patient made fully aware of the brittle nature of the diabetes. Diabetic ketoacidosis is rare in patients with chronic pancreatitis as there is usually enough residual insulin secretion to prevent release of fatty acids from adipose tissue and their subsequent metabolism in the liver to produce ketone bodies.
Miscellaneous features
Nausea, vomiting, and dyspepsia are common. Anorexia may be present but is often a reflection of the fact that the patient is afraid to eat rather than true loss of appetite. Weight loss and malnutrition with vitamin deficiencies are particularly common in alcohol-induced chronic pancreatitis. Gastrointestinal, retroperitoneal, and intraperitoneal bleeding may arise from erosion of pancreatic and peripancreatic vessels involved in inflammation. Bleeding from peptic ulceration, gastritis, and duodenitis is not rare and oesophageal varices may be present as a consequence of splenic vein thrombosis and segmental portal hypertension.
Pleural effusion and ascites are more often complications of acute pancreatitis but both can occur in chronic pancreatitis.
Associated disease
While evidence of biliary obstruction is commonly present on liver biopsy in patients with chronic pancreatitis, hepatic cirrhosis affects less than 5 per cent of patients. There may be a link between inflammatory bowel disease and chronic pancreatitis but pancreatitis may simply result from inflammation at the papilla of Vater. Primary sclerosing cholangitis is linked to exocrine pancreatic insufficiency but its relationship to chronic pancreatitis per se is uncertain. An association between chronic pancreatitis and pancreatic cancer remains speculative but the two can coexist and pose diagnostic difficulty. The increased incidence of non-pancreatic cancer in patients with chronic pancreatitis almost certainly reflects the smoking habits and alcohol consumption of this patient population.
DIAGNOSIS
Advanced chronic pancreatitis is seldom difficult to diagnose in the presence of calcification, malabsorption, and diabetes. The diagnosis of early disease is more difficult, and suspicions raised by the history are seldom accompanied by findings on physical examination. Tests of pancreatic function are of limited value given the functional reserve of the pancreas, and more reliance is now placed on imaging investigations which can detect changes in pancreatic morphology.
Tests of pancreatic exocrine function
An ideal test would detect pancreatic insufficiency before malabsorption became apparent, have a high specificity, and distinguish pancreatitis from pancreatic cancer. None of the tests available fulfils these criteria.
The secretin–cholecystokinin test
This is probably the best of the available tests of pancreatic function but it is now used rarely or not at all in many centres. Gastric contents are removed via a gastric tube and a duodenal tube is used to aspirate pancreatic secretions after stimulation by exogenous hormones. A marker such as C-polyethylene glycol may be used to ensure that at least 85 per cent of duodenal juice is recovered. Output of bicarbonate, trypsin, and amylase can be measured, but lipase determination is difficult technically. The test has a sensitivity of 75 to 90 per cent but attempts to improve its specificity beyond 80 to 90 per cent simply lower sensitivity.
Tubeless indirect tests of pancreatic function
These remain in use although they are less sensitive and specific than the secretin cholecystokinin test. In the bentiromide test, a synthetic tripeptide (N-benzoyl- l-tyrosyl- p-aminobenzoic acid) is given orally. In the presence of pancreatic chymotrypsin the tripeptide is cleaved to release p-aminobenzoic acid, which is absorbed from the small bowel, partially conjugated in the liver, and excreted in the urine. The pancreatolauryl test uses the same principle in that the ester fluorescein dilaurate is hydrolysed by pancreatic arylesterases to release fluorescein which is absorbed and excreted in the urine where it is detected spectrophotometrically. In patients with chronic pancreatitis judged to be severe on the basis of the secretin–cholecystokinin test, tubeless tests have a sensitivity of about 70 per cent; in mild to moderately severe disease this falls to below 50 per cent.
Other tests
Many have been described but not fully evaluated. The secretion of pancreatic lipase can be assessed by blood radioactivity levels after ingestion of C-labelled triolein or by measuring breath CO. Lactoferrin concentration (and lactoferrin: lipase ratio) in pancreatic juice is increased in chronic pancreatitis, and plasma pancreatic polypeptide levels have been correlated with enzyme secretion during fasting and after pancreatic stimulation. Serum trypsin-like immunoreactivity and pancreatic isoamylase concentrations during fasting are also lower in chronic pancreatitis. None of these tests is used in routine clinical practice.
Tests of pancreatic endocrine function
Development of diabetes is assessed by testing for glycosuria, random blood glucose determinations, and an oral glucose tolerance test.
Imaging investigations
Ultrasonography
This is inexpensive, non-invasive and avoids ionizing radiation, and is the first investigation to be undertaken when chronic pancreatitis is suspected. Pancreatic size is assessed; in the early stages inflammation causes enlargement with an irregular outline, while in the advanced stages atrophy may ensue. There is often a heterogeneous pattern with increased echogenicity, and calculi charteristically produce very bright echoes. Pseudocysts and abscesses are readily detected. The pancreatic duct is frequently discernible and a diameter of more than 2 mm is regarded as abnormal. In normal individuals, injection of secretin produces an increase in duct diameter. In patients with chronic pancreatitis this may be prevented by surrounding fibrosis. Useful information may be obtained regarding primary disease in neighbouring organs, such as the liver and biliary system, which may be responsible for the patient's symptoms, and complications of pancreatitis affecting these organs, such as biliary obstruction and splenic infarction may be detected. Ultrasonography can also be used to guide percutaneous fine needle sampling for cytology or histology when the nature of a pancreatic mass lesion is uncertain, and can facilitate the aspiration or drainage of pseudocysts and abscesses. Percutaneous needle pancreatography can also be carried out under ultrasonographic control and Doppler ultrasonography can now be used to diagnose portal and splenic vein occlusion.
CT scanning
Since this investigation is more expensive than ultrasonography and requires exposure to ionizing radiation, it is not normally used as a first line investigation. It is, however, more sensitive than ultrasonography in the diagnosis of chronic pancreatitis (sensitivity 74 to 90 per cent as opposed to 60 to 70 per cent) and at least as specific (84 to 100 per cent versus 80 to 90 per cent respectively). Pancreatic size, density, and outline are readily assessed and CT scanning is the best method for detecting calculi. Pancreatic duct changes on CT scanning correlate well with those seen at endoscopic retrograde pancreatography, and duct size is best assessed after intravenous injection of contrast material. CT scanning also provides useful information about neighbouring organs and may distinguish between pancreatic cancer and chronic pancreatitis.
Endoscopic retrograde pancreatography
This is invasive, expensive, requires exposure to ionizing radiation and can give rise to complications which, on rare occasions, prove fatal. The Cambridge classification of radiological changes is generally accepted and this investigation is widely regarded as the most accurate means of diagnosing chronic pancreatitis and distinguishing it from pancreatic cancer. Endoscopic retrograde pancreatography helps to define the extent and distribution of chronic pancreatitis and outlines the duct system. This information may prove invaluable in the selection of operative procedure if surgery is to be undertaken. Useful information may also be provided about involvement of the biliary tree. However, technical reasons may prevent cannulation of the pancreatic duct and ductal narrowing or obstruction may prevent full visualization of the duct system when contrast is injected. In such cases, ultrasonography and CT scanning may provide some of the missing information, but ultrasonographically guided percutaneous pancreatography or operative pancreatography may be needed to provide a comprehensive picture of duct morphology.
Some patients with symptoms and other evidence of chronic pancreatitis have little or no abnormality visible on endoscopic retrograde pancreatography. This has given rise to the controversial concept of minimal change pancreatitis.
Angiography and venography
Selective angiography and portal venography may be needed in selected patients, notably when portal or splenic vein thrombosis is suspected. Other imaging methods being evaluated at present include magnetic resonance imaging and endoscopic ultrasonography.
Other investigations
Assessment of nutritional status, full blood count, and liver function should be performed periodically, and it is worth carrying out random blood alcohol samples when continuing alcohol abuse is suspected.
Recommendations in diagnosis
The value of a good history cannot be over emphasized. When there is overt steatorrhoea, pancreatic function tests are unnecessary and one should proceed to ultrasonography and/or CT scanning. Even in the absence of steatorrhoea, pancreatic function tests (such as the secretin–cholecystokinin test or tubeless tests) are of dubious value. They are certainly useless in distinguishing between chronic pancreatitis and pancreatic cancer, and guided fine needle aspiration or biopsy should be undertaken whenever cancer is suspected. The presence of pancreatic calcification does not exclude cancer and pancreatitis and cancer may coexist. Endoscopic retrograde cholangiopancreatography should be considered once the pancreas has been imaged by ultrasonography and/or CT scanning, particularly when the diagnosis remains in doubt, duct morphology has not been defined, and surgery is contemplated.
TREATMENT
The diagnosis of chronic pancreatitis is not in itself an indication for surgery. Some patients have little or no pain, while in others pain can be controlled without recourse to opiates. Surgery does not restore exocrine and endocrine function; indeed pancreatic resection may cause marked deterioration and precipitate the onset of diabetes. Chronic pancreatitis is often difficult to manage and when there are problems arising from alcoholism, social deprivation, and personality disorders it may be impossible to disentangle cause from effect. Good rapport and mutual trust are essential for successful long-term management and require frequent outpatient consultation with periods of inpatient assessment if appropriate.
Medical management
Pain
Effective pain relief is usually the most difficult aspect of management. Patients vary considerably in pain threshold and pain perception and many are already taking opiates when first referred for surgical consultation. They should be encouraged to abstain from alcohol, take frequent small meals rather than infrequent large ones, and avoid overtly fatty foods. Liquid meals and elemental diets are no longer prescribed in attempts to reduce pancreatic stimulation: liquids augment the gastric phase of pancreatic secretion while elemental diets stimulate cholecystokinin release. Pancreatic extracts are used to treat steatorrhoea and probably reduce pain by providing intraluminal trypsin which blocks release of cholecystokinin from the duodenal mucosa. Histamine H receptor antagonists such as cimetidine or ranitidine may prevent or eradicate duodenitis and peptic ulceration, and theoretically reduce pancreatic stimulation by diminishing duodenal release of secretin.
Analgesics must be prescribed carefully, given the long-term nature of the problem and risk of opiate addiction. Unfortunately, non-opioid drugs such as aspirin and paracetamol are more effective at relieving musculoskeletal pain than visceral pain, and opiates are frequently essential. All opioid drugs may cause drowsiness, nausea and vomiting, and constipation (although this may be regarded as beneficial in patients with steatorrhoea). Sublingual bruprenorphine (200,400 mgr;g every 6-8 h), and oral dihydrocodeine tartrate (30 mg every 4-6 h) or pentazocine (25,100 mg every 3-4 h after food) may give satisfactory pain control without recourse to injection. More severe exacerbations of pain may require injection of morphine (10,30 mg) or pethidine (50,100 mg) and long-lasting severe pain may need oral pethidine (50,150 mg every 4 h) or long-acting morphine preparations (such as morphine sulphate, MST Continus, 10,60 mg twice daily). Whenever such powerful opioid drugs are having to be prescribed frequently and/or continuously, serious consideration must be given to surgery.
The sensory sympathetic nerves which transmit pain impulses from the pancreas synapse in the coeliac ganglion on their way to the splanchnic nerves. Attempts to relieve the pain of chronic pancreatitis by CT guided injection of alcohol in and around the coeliac plexus have had extremely variable results and at best give pain relief for a few months. Coeliac blockade is no longer used in most centres.
Pancreatic exocrine insufficiency
In theory, provision of exogenous lipase supplements with every meal should readily eliminate steatorrhoea. In practice steatorrhoea may be difficult to abolish as gastric acid destroys up to 90 per cent of such enzyme activity by the time food reaches the distal duodenum. Antacids or H-receptor antagonists were once used to combat this problem. However, modern pancreatic exocrine supplements (e.g. Creon; one capsule of which contains 8000 units lipase, 9000 units amylase, and 210 units protease) consist of enteric coated granules of pancreatic extract contained in an outer gelatin capsule. The gelatin dissolves with a few minutes in the acidic stomach whereas the granules break down in the duodenum when luminal pH rises above pH 5.5. Microsphere preparations are more expensive than simpler preparations of pancreatic extracts but are well tolerated. The initial dose is 1 to 2 capsules with meals, rising to 5 to 15 capsules each day. If steatorrhoea persists, H-receptor antagonists should be prescribed. If steatorrhoea is still troublesome, alternative explanations (such as bacterial overgrowth in the small intestine or bile salt insufficiency) should be considered. If all else fails, fat intake should be reduced to below 40 g per day and the diet supplemented with medium chain triglyceride oil which requires less lipase for cleavage.
Diabetes mellitus
Insulin is needed if diabetes mellitus develops. As indicated earlier, the risk of hypoglycaemia is much greater than that of ketoacidosis.
Other measures
Nutritional status requires careful supervision. An adequate intake of calories and vitamins is essential, particular attention being paid to intake in alcoholic patients. Recent suggestions that antioxidant supplements may be of value in pain relief require further evaluation.
Surgical management
Pain is the most common indication for surgery. The decision to operate is only taken after full trial of medical therapy and comprehensive evaluation of the patient and his domestic situation. The patient must not be talked into undergoing surgery and should be aware that many patients do not require surgery and that pain may gradually abate with time. He should have no illusions about the prospects following operation. Exocrine and endocrine function do not return to normal and arrest or slowing of functional deterioration is a realistic goal if a drainage operation is proposed. If resection is required, brittle lifelong diabetes may be precipitated and is inevitable after total pancreatectomy. Surgery does not always cure or improve pain and, even after total pancreatectomy, up to 20 per cent of patients still experience pain. A realistic guideline is that some 70 per cent of patients are still pain free or improved 5 years following operation. Many surgeons refuse to operate on alcoholics who are still drinking, and all agree that the prognosis after operation is much better in those who abstain.
Other factors which may indicate the need for surgery are the development of complications (see below) and uncertainty regarding the presence of pancreatic cancer.
Drainage operations
Longitudinal pancreaticojejunostomy
This is the standard operation when the pancreatic duct system is dilated to more than 7 to 8 mm in diameter. A complete pancreatogram is advisable and operative pancreatography (using needle puncture of the duct in the body or tail of the pancreas or transduodenal cannulation) may be needed if endoscopic retrograde pancreatography has been unsuccessful. The pancreas is exposed fully by division of the gastrocolic ligament and the pancreatic duct is opened fully along its length. A minimum length of pancreatico-jejunal anastomosis of 10 cm is advised for long-term patency; the longer the anastomosis the better. Calculi are removed from the duct system and a Roux loop of proximal jejunum is brought through a window in the transverse mesocolon and anastomosed to the pancreatic duct in side-to-side manner. It is not necessary to remove the spleen during this operation. The Roux loop is orientated so that its end lies on the tail of the pancreas; this allows the same loop to be used for anastomosis to the biliary system should biliary obstruction develop. If biliary obstruction is already present, a side-to-side hepaticojejunostomy is undertaken at the time of pancreaticojejunostomy. Pseudocysts can also be drained into the Roux loop.
Alternatives to longitudinal pancreaticojejunostomy include caudal pancreaticojejunostomy and pancreaticogastrostomy. The former operation involves anastomosing a loop of jejunum to the cut surface of the pancreas after removing the tail; it is now obsolete. Pancreaticogastrostomy still has adherents but there is no objective evidence for its superiority over the conventional operation.
Transduodenal sphincteroplasty
This may be performed with or without removal of calculi from the pancreatic duct, and is of debatable value. Benefit has been claimed in small series of selected patients but for the vast majority it seems inherently unlikely that pancreatitis arises from obstruction of the terminal portion of the duct and that sphincteroplasty will give long-term success. This said, there is now a growing number of reports of treatment of chronic pancreatitis by endoscopic papillotomy or balloon dilatation, accompanied in some cases by insertion of a stent and destruction of calculi by extracorporeal shock wave lithotripsy. The long-term results are yet to be defined but the approach may defer or avoid operation in selected patients.
Accessory sphincteroplasty
Endoscopic and surgical means can be used in this manner to treat chronic pancreatitis associated with pancreas divisum. In the main, the results have been disappointing, calling into question the significance of pancreas divisum as an aetiological factor.
Resectional surgery
Distal pancreatectomy
Removal of 40 to 95 per cent of the pancreas was at one time popular, but its popularity waned with the realization that many patients still had pain after surgery and that marked exocrine and endocrine insufficiency often resulted. There is still a place for distal resection, conserving the spleen if possible, in patients in whom chronic inflammation appears to be confined to the distal pancreas.
The Whipple resection
This is regarded by many as the standard operation when the duct system is not sufficiently dilated for pancreaticojejunostomy. The head of the gland is often the most inflamed part, the operation conserves useful exocrine and endocrine function by retaining the body and tail, any biliary tract obstruction is dealt with, and the risk of leaving occult cancer in the head of the pancreas is avoided. The gallbladder should be removed to avoid subsequent gallstone formation and the jejunum is anastomosed to the common hepatic duct rather than common bile duct to reduce the risk of ischaemic breakdown of the anastomosis. Opinions vary as to whether antrectomy should be performed or whether the first part of the duodenum should be transected with preservation of the pylorus and entire stomach. One variant preserves the stomach and entire duodenum. The case for pylorus-preserving and duodenum-preserving pancreaticoduodenectomy rests on retention of more normal gastrointestinal physiology and function, but has yet to be accepted universally. Truncal vagotomy was once an integral part of the Whipple operation but the risk of marginal ulceration is now so small that few surgeons divide the vagi. Most surgeons attempt to retain exocrine function by anastomosing the cut surface of the remaining pancreas to the jejunum. Others attempt mucosa-to-mucosa apposition between the pancreatic duct and jejunum while some doubt the value of attempting to retain exocrine function and staple the transected pancreas or occlude its duct system.
Total pancreatectomy
This is a last resort given the inevitable diabetes and steatorrhoea which result. Most patients coming to total pancreatectomy have already failed to benefit from lesser surgical procedures and it must be re-emphasized that some 20 per cent of patients still complain of pain following total extirpation. Pylorus-preserving and duodenum-preserving forms of total pancreatectomy have been used. One advantage sometimes claimed for total pancreatectomy is that it avoids the need for anastomosis between residual pancreas and jejunum. However, this anastomosis is not as dangerous in chronic pancreatitis as it is in cancer surgery. In chronic pancreatitis the gland is fibrous and firm rather than friable, and the reduced exocrine secretory capacity lowers the risk of leakage and fistula formation.
Other operations
Attempts to relieve pain by excision of the coeliac ganglion and other forms of neurectomy have generally proved disappointing. Left transthoracic splanchnicectomy and bilateral truncal vagotomy has recently been advocated but requires further evaluation.
Comparison of surgical procedures
The ideal operation should have no operative mortality, provide permanent relief of pain, and conserve pancreatic exocrine and endocrine function. Additional aims would be minimal interference with normal digestive function and elimination of the risk of failing to remove occult pancreatic cancer. All operations should now carry a risk of operative mortality near to zero if performed in specialist hands. Pancreaticojejunostomy is safer than resection and offers a good prospect of pain relief with maximal conservation of pancreatic function. Unfortunately only some 20 to 30 per cent of patients have a duct system which is sufficiently dilated for this operation. It remains to be seen whether endoscopic procedures will retain a place in management as an alternative to surgical drainage. Resection has become safer with improved operative and perioperative care but still carries a greater risk than drainage surgery. Tissue planes are frequently destroyed by inflammation, and operative damage to major vessels (particularly the portal and superior mesenteric vein) poses a real hazard. The greater the resection, the greater the risk of exocrine and endocrine insufficiency. Distal pancreatectomy is indicated when disease is confined to or is maximal in the body and tail and can be combined with drainage of the duct system of the remaining pancreas. The Whipple operation (or its variants) is now used in the majority of patients, while total pancreatectomy is reserved for patients in whom lesser procedures fail to relieve pain.
Management of complications
Pseudocysts
The availability of ultrasonography and CT scanning has improved our understanding of the natural history of pseudocysts in chronic pancreatitis. As a general rule pseudocysts which exceed 5 cm in diameter and which have been present for more than 6 weeks are unlikely to resolve spontaneously. When treatment is needed, internal drainage is safer and more effective than external drainage or resection. Biopsy of the pseudocyst wall is always advisable at surgery as cystic pancreatic neoplasms can cause confusion in diagnosis. A Roux loop of jejunum is usually recommended for internal drainage although on occasions the stomach or duodenum may be used. If a fluid collection proves to be an abscess, external drainage is advisable. False aneurysms can masquerade as pseudocysts and erosion of major vessels may cause bleeding into a pre-existing pseudocyst. It is always advisable to have a high index of suspicion, sample the cyst contents by needling before incision, and consider preoperative angiography when any doubt exists.
Biliary obstruction
Transient jaundice and elevation of serum alkaline phosphatase levels is common in chronic pancreatitis. Ultrasonography and, if necessary, endoscopic retrograde cholangiopancreatography can be used to define biliary morphology, and liver biopsy is indicated if there is long-standing derangement of liver function. However, alcoholic hepatitis and cirrhosis are surprisingly uncommon, even in patients with chronic alcohol-associated pancreatitis, secondary biliary cirrhosis is rare, and most patients merely have histopathological evidence of biliary obstruction with or without cholangitis. When the bile duct is narrowed a long segment of stenosis in the retropancreatic bile duct is usually found although hour-glass constriction or lateral displacement may occur. Bile duct obstruction in chronic pancreatitis is usually due to fibrosis of the surrounding pancreas rather than acute inflammation or pressure from a pseudocyst. Given the long-term risk of cholangitis and secondary biliary stenosis, fibrotic narrowing of the bile duct requires surgical treatment. Such surgery to relieve biliary obstruction is needed in 10 to 20 per cent of patients with chronic pancreatitis. Resection of the head of the pancreas may also be indicated because of pain, but if resection is not needed biliary drainage can be achieved by hepaticojejunostomy or choledochoduodenostomy. Cholecystojejunostomy is not recommended as it fails to ensure long-term bile flow and leaves the patients at risk of developing gallbladder disease. Temporary drainage can be obtained by endoscopic biliary stenting if there is a need to avoid operation.
Gastrointestinal obstruction
Transient duodenal obstruction may complicate an exacerbation of inflammation in the head of the pancreas. Chronic fibrotic obstruction is a relatively rare complication of chronic pancreatitis (2 per cent of cases) and endoscopy is essential to exclude peptic ulceration, Crohn's disease, and pancreatic or periampullary cancer. Persisting fibrotic obstruction demands surgical relief. Gastroenterostomy is used if duodenal obstruction is the sole indication for surgery or when pancreaticojejunostomy is also needed. Where there is painful disease in the head of the pancreas and no duct dilatation, the Whipple operation is used.
Colonic obstruction is rare; when it occurs it is usually transient, involving the distal colon or splenic flexure. Operation is only indicated if obstruction persists for more than a few weeks.
Haemorrhage
Arterial haemorrhage may result from necrosis of the vessel wall in areas of inflammation and pseudocyst formation. The splenic, gastroduodenal, pancreaticoduodenal, pancreatic, gastric, and hepatic arteries may be involved, in order of frequency. Rupture can result in pseudoaneurysm formation and bleeding can occur into the pancreatic duct, pseudocyst, retroperitoneal tissues, or peritoneal cavity. Endoscopy and selective angiography are useful investigations and although surgery is usually indicated, therapeutic embolization may be life saving in high risk patients.
Thrombosis
Thrombosis of the splenic vein, and less frequently of the portal and superior mesenteric vein, can be asymptomatic. Alternatively, the patient may bleed from gastric and oesophageal varices or present with a large spleen, leucocytopenia, and thrombocytopenia. Haemorrhage from colonic varices is exceptional. When variceal bleeding occurs after splenic vein occlusion, splenectomy is the treatment of choice. A preoperative arteriogram and venogram is advisable, partly to define the problem and partly to embolize the splenic artery to reduce collateral bleeding at operation.
Pleural effusion, fistula, and pancreatic ascites
Leakage of pancreatic juice from rupture of a pseudocyst or inflammation and necrosis of the pancreatic duct may result in rapid development of ascites and pleural effusions. The diagnosis is confirmed if the fluid protein content exceeds 25 g/l and if the amylase concentration of the fluid exceeds that of the serum. Conservative treatment with parenteral nutrition and the somatostatin analogue (to reduce pancreatic secretion) may be employed initially but is seldom successful. Surgery should not be postponed beyond 2 to 3 weeks. Preliminary endoscopic retrograde pancreatography is invaluable to define the site of leakage. Leaks in the tail are usually treated by distal pancreatectomy, those elsewhere by pancreaticojejunostomy.
Internal pancreatic fistulae involving the gut may not require surgical intervention and are often discovered as incidental findings at endoscopic retrograde pancreatography. Pancreaticocutaneous fistulae frequently follow external drainage of pseudocysts or may complicate pancreatic surgery. They often respond to conservative measures but surgical resection of the involved pancreas may be needed.
PROGNOSIS
The prognosis in chronic pancreatitis depends on the frequency and severity of the attacks, need for surgery, and the development of complications, notably diabetes. Alcoholics who fail to stop drinking undoubtedly fare worse than non-alcoholics and alcoholics who abstain. Given the importance of alcohol as an aetiological factor and its associated personality and social upset, it is hardly surprising that patients with chronic pancreatitis have a lower life expectancy than the general population. The complications of the disease, drug addiction, depression, brittle diabetes, malnutrition, and increased risk of non-pancreatic cancer combine to reduce longevity. Reported survival rates vary greatly according to the nature of the series of patients studied. The cumulative survival rate based on life table analysis in alcoholics with or without surgery is about 50 per cent at 20 to 24 years from onset of the disease; non-alcoholic patients have survival rates which are some 20 per cent higher.
FURTHER READING
Banks PA. Medical strategy in chronic pancreatitis. In: Carter DC, Warshaw AL, eds. Pancreatitis. Edinburgh: Churchill Livingstone, 1990; 133;47.
Sarles H, Bernard JP, Gullo L. Pathogenesis of chronic pancreatitis. Gut 1990; 31: 629;32.
Singh SM, Reber HA. The pathology of chronic pancreatitis. World J Surg 1990; 14: 2;10.
Stone HH, Chauvin EJ. Pancreatic denervation for pain relief in chronic alcohol associated pancreatitis. Br J Surg 1990; 77: 303;5.
ГОУ ВПО «Смоленская государственная медицинская академия
Федерального агентства по здравоохранению и социальному развитию»
МЕТОДИЧЕСКИЕ УКАЗАНИЯ ДЛЯ СТУДЕНТОВ
ПО ДИСЦИПЛИНЕ хирургические болезни
PANCREATIC CARCINOMA
Составитель доц. А.Ю.Некрасов
Методические указания утверждены на методическом совещании кафедры госпитальной хирургии (протокол № 2 от 6 октября 2008 г.)
Зав. кафедрой______________(проф. С.А.Касумьян)
2008 г.
THEME: CANCER OF THE EXOCRINE PANCREAS
QUESTIONS FOR HOMEWORK:
Anatomy of pancreas
Risk factors in pancreatic cancer
Pathologic features
Basic symptoms of cancer pancreas
Clinicopathologic staging
Diagnosis and clinical staging
Palliative intervention
Resectional therapy
ANATOMY OF THE PANCREAS
The pancreas occupies a retroperitoneal position in the abdomen. It lies posterior to the stomach and lesser omentum. It extends obliquely from the duodenal C-shaped loop to a more cephalad position in the hilum of the spleen.
The normal adult pancreas varies in weight from 75 to 125 g. It is in length is from 10 to 20 cm. In the anteroposterior axis, the pancreas is the thickest at the head (1.5 to 3.5 cm) and it is the thinnest at the tail (0.8 to 2.5 cm). The gland has a distinctive yellow-tan-pink color and is has some lobules. The pancreas is covered by peritoneum anteriorly. Posteriorly it lies the adjacent structure of the pancreas are the inferior vena cava, the right renal vein, the aorta at the level of the first lumbar vertebra, the superior mesenteric vessels and the splenic vein.
The gland is divided into four portions: the head, neck, body, and the tail. That portion of the pancreas anterior to the superior mesenteric and portal veins is called the neck of the gland. The head of the gland extends to the right of the neck and lies within the bordes of the duodenal C-shaped loop; it includes the posteroinferior extension arising from the ventral primordium called the uncinate process. The uncinate process extends posterior to the superior mesenteric vein and ends at or extends beyond the right margin of the superior mesenteric artery. The body of the pancreas lies immediately to the left of the neck. The tail of the pancreas extends to the left of the body into the splenic hilum.
An extensive arterial system originating from multiple sources supplies the pancreas. The head of the pancreas is intimately associated with the second portion of the duodenum, and these two structures are both supplied by two arterial arcades known as the anterior and posterior pancreaticoduodenal arteries. These arteries originate from the superior and inferior pancreaticoduodenal vessels as branches of the celiac axis and superior mesenteric artery, respectively. The blood flow to the body and tail of the pancreas occures through a more variable complex of arteries. The distal body and tail of the pancreas are supplied by branches of the splenic and left gastroepiploic arteries, with the largest being the dorsal pancreatic and transverse pancreatic arteries.
The venous drainage of the pancreas is closely connected with it’s arterial anatomy. Veins draining the pancreatic parenchyma eventually terminate into the portal vein, which arises posterior to the neck of the pancreas the splenic and superior mesenteric veins.
Multiple lymph node groups drain the pancreas. From the head of the gland, nodes in the pancreaticoduodenal groove communicate with subpyloric, portal, mesocolic, mesenteric, and aortocaval nodes. Lymphatic vessels in the body and tail of the pancreas drain to retroperitoneal nodes in the splenic hilum or to celiac, aortocaval, mesocolic, or mesenteric nodes.
A dual sympathetic and parasympathetic innervation achivales the pancreas. Preganglionic sympathetic axons arise from cell bodies within the thoracic sympathetic ganglia. From these structures, postganglionic sympathetic fibers traverse retroperitoneal tissue to innervate the pancreas and serve as the principal pathways for pain of pancreatic origin. This sympathetic pathway is the target during splanchnicectomy for the relief of pain of pancreatic origin.
The parasympathetic innervation of the pancreas preganglionic fiber cell bodies that reside within the vagal nuclei and travel through the posterior vagal trunk. These axons travel through the celiac plexus to terminate in parasympathetic ganglia within the pancreatic parenchyma. Postganglionic parasympathetic fibers to innervate the pancreatic islets, acini and ducts, and they serve an exclusively efferent function.
RISK FACTORS IN PANCREATIC CANCER
The demographic features of pancreatic cancer are presented (Table 1). Factors that are related to an increased risk of pancreatic cancer include advancing age, black race, male gender and Jewish ethnicity The importance of genetic and host alterations in the development of pancreatic cancer is most apparent in the six genetic syndromes that are associated with an increased risk for the development of pancreatic cancer. These syndromes are hereditary nonpolyposis colorectal cancer (HNPCC), familial breast cancer associated with the BRCA2 mutation, Peutz-Jeghers syndrome, ataxia-telangiectasia syndrome, familial atypical multiple mole melanoma syndrome (FAMMM) and hereditary pancreatitis. Although an apparent association between diabetes and pancreatic cancer has been reported by many investigators, the data are inconsistent and the bulk of the data indicate no consistent association, except when cases are included in which diabetes was diagnosed within between 1 and 5 years of the cancer diagnosis. The relationship between chronic pancreatitis and pancreatic cancer has been considerably stadied, and several studies have suggested that chronic pancreatitis is associated with an increased risk of the subsequent pancreatic cancer development. Other low-level risk factors include cystic fibrosis, the use of estrogen hormones, and pernicious anemia.
Investigations clearly indicate that cigarette smoking increases the risk of cancer of the pancreas. As concerns nutrition and diet, an association appears to exist between pancreatic cancer and increasing total energy intake, carbohydrate, cholesterol, meat, and other nutrient intakes. Some workers are believed to be at increased risk for pancreatic cancer including chemists, coal gas workers, and many others.
TABLE 1. Risk Factors in Pancreatic Cancer
Factors |
Increased Risk |
Possible Risk |
Unproven Risk |
Decreased Risk |
Demographic |
Advancing age Black race Male gender Jewish ethnicity |
Geography |
Socioeconomic status Migrant status |
|
Host |
HNPCC Familial breast cancer Peutz-Jeghers Ataxia-telangiectasia FAMMM Hereditary pancreatitis |
Diabetes Chronic pancreatitis Endocrine tumors Cystic fibrosis Sex hormones |
Peptic ulcer surgery Cholecystectomy |
Tonsillectomy Allergic disorders |
Environmental |
Tobacco |
Diet Occupation |
Alcohol Coffee Radiation |
|
Abbreviations: FAMMM, familial multiple mole melanoma; HNPCC, hereditary nonpolyposis colorectal cancer.
PATHOLOGIC FEATURES
Cancers of the pancreas can be classified into primary, meta-static, or systemic. Primary cancers arise in the pancreas. They can show either exocrine or endocrine differentiation. Metastatic cancers originate elsewhere and spread hematogenously or by lymphatics to the pancreas. Systemic malignancies are derived from the blood or lymph nodes and by definition simultaneously involve multiple sites. One such sites may be the pancreas. Ductal adenocarcinoma is an epithelial, nonendochne solid neoplasm that is the most common primary pancreatic malignancy.
Ductal adenocarcinoma accounts for more than 80% of all primary pancreatic cancers. Approximately 65% of ductal adenocarcinomas arise in the head, neck, or uncinate process of the pancreas. 15% them originate in the body and tail and 20% diffusely involve the entire gland. Grossly, ductal adenocarcinomas are white-yellow, poorly defined, firm masses that often obstruct and dilate the adjacent distal common bile duct or main pancreatic duct. In many cases the tumor arises in close proximity to the genu (or knee) of the main pancreatic duct. Microscopically ductal adenocarcinomas contain infiltrative glands of various shapes and sizes surrounded by dense reactive fibrous tissue. Many ductal adenocarcinomas infiltrate into vascular spaces, lymphatic spaces, and perineural spaces. At the time of discovery, most ductal adenocarcinomas have metastasized into peripancreatic lymph nodes. In addition to lymph node metastases, by the time of the patient's death, pancreatic ductal adenocarcinomas have frequently metastasized to the liver (up to 80% of cases), peritoneum (60%), lungs and pleura (50 to 70%), and adrenal glands (25%).
Received data suggest that histologically identifiable precursor lesions progress to infiltrating ductal adenocarcinoma of the pancreas. These abnormal ductal structures contain a mucin-producing proliferative epithelium with varying degrees of cytologic and architectural atypia. These lesions can be found adjacent to invasive ductal adenocarcinoma in resected glands. These ductal lesions display some of the same genetic changes that infiltrating adenocarcinomas display.
Adenosquamous carcinomas are a rare variant of ductal adenocarcinomas that show both glandular and squamous differentiation. Typically the squamous features predominate, although careful histologic examination almost always reveals some glandular component. The biological behavior of adenosquamous carcinoma appears similar to that of typical ductal adenocarcinoma, although some investigators have found that adenosquamous varieties have a particularly poorer prognosis.
Acinar cell carcinoma is a rare malignant tumor of the pancreas that may be large when discovered. These tumors have a distinct histologic appearance and occasionally have an unusual clinical presentation. Although most patients with acinar cell carcinoma have typical biliary or gastrointestinal obstruction because of the tumor, up to 20% of patients also experience the development of subcutaneous fat necrosis, an erythema nodosum-like rash, peripheral eosinophilia, and polyarthralgia. Acinar cell carcinomas are typically smooth, fleshy, and lobulated, with occasional areas of hemorrhage or necrosis. Histologically, these tumors typically form acini, and their cells display eosinophilic, granular cytoplasm. Although prognostic data are limited, it appears that patients with acinar cell carcinoma fare better than patients with ductal adenocarcinoma. Other rare exocrine malignancies of the pancreas include giant cell carcinoma, giant cell carcinoma with osteoclast-like giant cells, and the rare childhood neoplasm, pancreatoblastoma.
CLINICOPATHOLOGIC STAGING
TABLE 2. UICC Staging of Pancreatic Cancer
Stage grouping |
T |
N |
M |
5-year survival |
Stage I |
T1 or T2 |
N0 |
M0 |
20-40% |
Stage II |
T3 |
N0 |
M0 |
10-25% |
Stage III |
Any T |
N1 |
M0 |
10-15% |
Stage IV |
Any T |
Any N |
M1 |
0-8% |
Tumor (T): T1 = Limited to pancreas T2 = Extension directly to duodenum, bile duct or peripancreatic tissues T3 = Extension directly to stomach, spleen, colon or adjacent large vessels
Lymph Nodes (N): N0 = No lymph node metastases N1 = Lymph node metastases
Distant Metastasis (M): M0 = No distant metastasis M1 = Distant metastasis
The most commonly used staging system for pancreatic cancer is the Union Internationale Contra Ie Cancer (UICC) system, which is based on tumor-node-metastasis (TNM) factors (Table 2). This relatively easy to use system allows stage classification by primary tumor extent (T), regional lymph node involvement (N), and the presence or absence of distant metastases (M), with the subsequent determination of four stage groupings. Other more complex staging classification systems have been proposed by various groups, but they have not gained widespread use.
DIAGNOSIS AND CLINICAL STAGING
Jaundice is the symptom an inital sign of pancreatic cancer. Jaundice occurs as result of the neoplasm, arising in the head of the pancreas and obstructing the intrapancreatic portion of the common bile duct. Weight loss, abdominal pain, and pruritus often come with jaundice. Weakness, alteration of bowel habits, and anorexia may also be present. Frequently the abdominal pain progresses and is characterized as an unremitting midepigastric pain with some radiation to the back. Occasionally pancreatic cancer occurs in an unusual manner. New-onset diabetes may be the first clinical feature in approximately 10% of patients. Acute pancreatitis may be the first sign of a pancreatic neoplasm and may cause partial obstruction of the pancreatic duct. Other symptoms found in a few patients include nausea and/or vomiting related to gastroduodenal obstruction. Mechanical obstruction at the level of the proximal duodenum can be caused by neoplasms in the head of the pancreas, or alternatively, cancers of the midbody of the pancreas may cause obstruction at the duodenojejunal junction.
At the time of the initial examination the most common physical finding is jaundice. Hepatomegaly and a palpable gallbladder may also be found. In advanced cases patients may have evidence of cachexia, muscle wasting, and a nodular texture to the liver consistent with metastatic disease. Other physical findings in patients with disseminated pancreatic cancer may include left supraclavicular adenopathy (Virchow node), periumbilical lymphadenopathy (Sister Mary Joseph nodes), and drop metastases in the pelvis encircling the perirectal region (Blumer shell).
Laboratory studies in patients with cancer of the head of the pancreas typically reveal elevated serum total bilirubin, alkaline phosphatase, and gamma-glutamyl transpeptidase, with mild elevations of the hepatic aminotransferases. Hepatitis serologic tests are often evaluated and are typically negative. In patients with localized cancer of the body and tail of the pancreas, early laboratory values are normal. Most patients with carcinoma of the pancreas have neither hyperamylacemia nor hyperlipasemia. In patients with marked jaundice, the coagulation parameters should be assessed because prolonged exclusion of the bile from the gastrointestinal tract leads to malabsorption of fat-soluble vitamins, with decreased hepatic production of vitamin K-dependent clotting factors.
Many diagnostic imaging modalities are available to assess patients with pancreatic cancer. Transabdominal ultrasonography is the most sensitive noninvasive test for detecting gallstones, an ever-present issue in an elderly patient who is jaundiced. Currently, however, high-quality spiral or helical CT scanning is the preferable noninvasive modality used for the diagnosis and staging of pancreatic cancer. Pancreatic cancer can usually be vevealed as an area of pancreatic enlargement by CT, typically as a hypodense focal lesion. In addition to the assessment of the primary tumor, the CT scan is used to evaluate major vessels adjacent to the pancreas for neoplastic invasion or thrombosis and to evaluate tumor spread to the liver, peripancreatic lymph nodes, or adjacent retroperitoneal structures. MRI, particularly the newer technique of ultrafast spin-echo MRI, to be more sensitive than older-generation CT scanning. Currently, CT is more cost effective and less time consuming, however, pancreatic MRI, particularly MRCP, is evolving rapidly, and advances in MRI technologies may lead to an increasing MRI role in pancreatic cancer.
ERCP allows direct imaging of the pancreatic duct, the site of origin of most pancreatic cancers. Although the sensitivity of ERCP for the diagnosis of pancreatic cancer is high, inaccuracies can arise, and complications can develop. Although no question exists that ERCP is reliable in confirming the clinical suspicion of pancreatic cancer, it is rarely necessary. With the current sophistication of spiral CT scanning or MRCR the routine practice of diagnostic ERCP in pancreatic cancer is unsupported. Diagnostic ERCP is probably best reserved for diagnostic dilemmas, such as the evaluations of patients with presumed pancreatic cancer and obstructive jaundice in whom no mass is evident on CT, symptomatic but nonjaundiced patients without an obvious pancreatic mass, or occasional patients with chronic pancreatitis in whom the development of a pancreatic cancer is suspected based on clinical deterioration.
Positron emission tomography (PET) scanning has been used in patients with pancreatic cancer. PET scanning uses the increased metabolism of glucose by pancreatic cancer cells as the basis of imaging. Some studies have suggested that PET scanning is more sensitive and specific than CT in the detection of small primary pancreatic tumors and in the assessment of hepatic and distant metastases. PET scanning has also been found to be helpful in assessing response to neoadjuvant chemoradiation. Further data are needed to clarify the role of PET scanning in pancreatic cancer.
As concerns the tissue diagnosis of pancreatic cancer, the use of percutaneous or endoscopic pancreatic biopsy in the diagnostic evaluation of a patient with suspected pancreatic cancer has advocates and vocal opponents. Although biopsy techniques are generally safe, potentially serious complications such as hemorrhage, pancreatitis, fistula, abscess, perforation, and death have been reported. Additionally concerns have been raised regarding tumor dissemination by the act of capsular rupture of the neoplasm. Biopsy appears to have no role in the evaluation of a patient who is a good-risk operative candidate and who has a clinically resectable pancreatic mass. A negative biopsy would not preclude operative exploration and resection; therefore, no reason exists to perform such a biopsy. However, a role exists for pancreatic biopsy or biopsy of distant metastases in liver or subcutaneous lymph nodes in patients who are considered a poor risk for major pancreatic resection. These patients may be candidates for palliative chemoradiation therapy after tissue diagnosis. Additionally, some forms of tissue diagnosis to document adenocarcinoma are mandatory in patients who are considered for various neoadjuvant protocols. Furthermore, biopsy may be considered in patients whose clinical presentation and imaging studies do not suggest pancreatic adenocarcinoma, but rather a less common entity such as pancreatic lymphoma.
Physical examination and routine imaging studies such as CT or MRI provide information regarding both diagnosis and staging. Additionally, three other techniques have been used for preoperative staging: angiography, endoscopic ultrasound, and laparoscopy Although some investigators have championed angiography to assist with the staging of patients with pancreatic cancer, others have believed that its performance is unnecessary because reliable information can be generated from modern high-quality spiral CT or MRI scans. Although some studies have shown that angiography adds to the reliability of CT, others have shown that modern imaging techniques can predict unresectability quite well, with little added information from angiography. Angiography can certainly document common vascular anomalies such as a replaced right hepatic artery originating from the superior mesenteric artery, or a stenotic celiac origin with hepatic arterial inflow being via cephalad flow from the gastroduodenal artery. Although these anomalies may be readily apparent to an experienced pancreatic surgeon, they may not be as apparent to occasional pancreatic surgeons.
Endoscopic ultrasound produces high-frequency images of the pancreas using the wall of the stomach and duodenum as an acoustic window. Although this method alone does not provide a tissue diagnosis, the development of endoscopic ultrasound-guided fine-needle aspiration makes the acquisition of tissue samples possible. Although the data are not consistent, some studies have suggested that endoscopic ultrasound is superior to the combination of CT plus ERCP for small tumors. However, although endoscopic ultrasound can be particularly useful in providing staging information regarding the extent of the primary tumor (T stage), it cannot reliably assess metastatic involvement of the liver, nor can it clearly establish distant lymph node involvement. Although endoscopic ultrasound appears promising as an adjunct in the staging of pancreatic cancer, it is highly operator dependent and certainly should not replace modern CT or MRI techniques.
Some investigators are proponents of routine staging laparoscopy in all patients with the diagnosis of potentially resectable pancreatic cancer. The rationale for the use of laparoscopy comes largely from data that indicate that between 20 and 40% of patients staged by other modalities such as CT, MRI, ERCP, and endoscopic ultrasound will be determined to have unanticipated peritoneal or liver metastases at the time of exploration. However, the inherent bias favoring the use of laparoscopy presumes an equivalence between nonoperative palliation and operative palliation in patients with pancreatic cancer. Simply, laparoscopic staging makes sense only if patients who are deemed to have unresectable tumors and are spared laparotomy can be effectively palliated nonoperatively. Degrees of expertise in the application of laparoscopy vary; some highly experienced groups perform more extensive laparoscopic evaluations than others. Extensive evaluation includes examination of the porta hepatis and mesenteric and celiac vessels and adds the use of laparoscopic ultrasound. Several studies of laparoscopy have shown that in patients with obstructive jaundice caused by tumors in the head of the pancreas, only 15 to 20% are found to have unexpected intraperitoneal metastases after routine staging studies, excluding laparoscopy. In contrast, patients with cancer of the body and tail of the pancreas may have unexpected peritoneal metastases after routine imaging studies in up to 50% of cases. Based on these data, staging laparoscopy appears to be supported for patients with cancer of the body or tail of the pancreas, because these tumors do not typically cause biliary or gastric outlet obstruction, and therefore these patients do not routinely require operative palliation for symptoms. However, because many surgeons believe that patients with cancers of the head of the pancreas are best palliated surgically by biliary-enteric bypass, gastrojejunostomy, and alcohol celiac nerve block, routine preoperative staging laparoscopy for patients with cancers of the head of the pancreas cannot be similarly supported.
PALLIATIVE INTERVENTION
Nonoperative management is appropriate in patients who are determined by staging studies to have distant metastases, unresectable local disease, or disseminated intra-abdominal tumor, or in patients with acute or chronic debilitating diseases that make anesthesia and surgery prohibitive. In these patients, a tissue diagnosis can usually be obtained through biopsy of distant metastases or local disease. The palliation of biliary obstruction is an important management issue in patients who present with obstructive jaundice. Biliary decompression can be achieved either by endoscopic or percutaneous transhepatic techniques in nearly all patients who are not candidates for surgical intervention. The methods of biliary decompression are well standardized, and a technical success rate in excess of 90% should be expected with either endoscopic or percutaneous approaches. The typical endoscopic approach involves deep biliary cannulation, manipulation of a guidewire above the malignant stricture, and the placement of a 7- to 10-French plastic endoprosthesis in the common bile duct to traversing the malignant tumor. Major procedure-related morbidity of the endoscopic approach is now less than 10%, and the major late complication is stent occlusion. The occurrence of such plastic stent occlusion has led most endoscopists to favor planned stent removal and replacement, typically at 3- to 6-month intervals. Metallic expandable endoprostheses have been developed for endoscopic placement, with most data suggesting a lower rate of stent occlusion. However, tumor ingrowth remains a problem with metallic stents and can cause late stent occlusion. Percutaneous approaches to a biliary decompression have similarly been well standardized. Diagnostic cholangiography first defines the site of bile duct obstruction, and subsequently a plastic drainage catheter can be manipulated across the malignant stricture into the duodenum. Subsequent management involves maintenance of internal-external drainage catheters or the percutaneous placement of either a plastic or a metallic endoprosthesis. Available data support the use of the endoscopic method as the primary method for nonoperative palliation of jaundice.
Pain associated with pancreatic cancer may result from tumor infiltration in the retroperitoneal celiac plexus, gastroduodenal obstruction, gallbladder distention, or increased pancreatic parenchymal pressure from pancreatic ductal obstruction. Such tumor-associated pain may be an incapacitating symptom of pancreatic cancer and is best treated with long-acting oral analgesics in appropriate doses, with the most common drug used being long-acting morphine sulfate. In patients who cannot take oral medications, topical analgesics worn as continuous-release cutaneous patches can be highly effective (fentanyl patches). Poorly controlled pain may require the expertise of pain management specialists.
Patients with high-grade duodenal obstruction secondary to pancreatic cancer generally require operative intervention for gastrojejunostomy. In patients with low-grade obstruction, modifications of available biliary-type metallic stents have led to scattered reports of success when these stents are placed endoluminally.
Operative management - palliative surgery for pancreatic cancer performed to relieve biliary obstruction, avoid or treat duodenal obstruction, palliate tumor-associated pain, and to improve the patient's quality of life. The three most common techniques of relieving biliary obstruction are choledochoduodenostomy, cholecystojejunostomy, and hepatico (choledocho) jejunostomy. The most common technique is biliary-enteric bypass to the jejunum performed either as an end-to-side hepaticojejunostomy or as a choledochojejunostomy, with the gallbladder removed before dissection and mobilization of the extrahepatic biliary tree. This method of biliary-enteric bypass is more common because it has the lowest incidence of recurrent jaundice and the lowest rate of postoperative cholangitis.
To avoid duodenal obstruction, prophylactic gastrojejunostomy can be done in patients with operative palliation of pancreatic cancer. This is typically performed as a retrocolic isoperistaltic loop gastrojejunostomy using the jejunum 20 to 30 cm beyond the ligament of Treitz and replacing the horizontal gastrotomy posterior in the most dependent portion of the greater curvature of the stomach. Vagotomy is not performed in this setting because it may contribute to delayed gastric emptying. Instead, routine acid secretory inhibition agents such as histamine H2-receptor antagonists or proton pump agents are used to prevent marginal ulceration.
Abdominal and back pain associated with unresectable pancreatic cancer often becomes the major debilitating symptom for the patient. Intraoperative chemical splanchnicectomy is designed to disrupt the transmission of afferent impulses to the celiac plexus. To date, the only prospective, randomized placebo-controlled study of intraoperative chemical splanchnicectomy indicated that the injection of 20 ml of 50% alcohol on either side of the aorta at the level of the celiac axis reduced postoperative pain scores and the need for postoperative analgesics. These data support the routine use of intraoperative chemical splanchnicectomy in all patients undergoing operation for unresectable pancreatic carcinoma.
RESECTIONAL THERAPY
For tumors of the head, neck or uncinate process pancreaticoduodenectomy is performed. The initial portion of the operative procedure is dedicated to the assessment of resectability. Hepatic metastases, serosal implants, and lymph node metastases outside the resection zone indicate unresectable disease. An extensive Kocher maneuver is performed, to elevate the duodenum and the head of the pancreas out of the retroperitoneum. The superior mesenteric artery is palpated to ensure that the vessel is not encased by tumor extending from the uncinate process. The porta hepatis is carefully assessed by mobilizing the gallbladder out of the gallbladder fossa and by following the cystic duct down to its junction with the common hepatic duct. Division of the extrahepatic biliary tree allows caudal retraction of the distal common bile duct and opens the plane to visualize the anterior portion of the portal vein. The plane between the neck of the pancreas and the anterior surface of the portal vein may be then developed, fully elevating the pancreatic neck from the vein. The division of the proximal gastrointestinal tract is typically performed 2 cm distal to the pylorus. Further steps in pancreaticoduodenal resection involve the division of the pancreatic neck and the final dissection of the head and uncinate process from the superior mesenteric vein, portal vein, and superior mesenteric artery. The most common reconstructive technique anastomoses the pancreas to the jejunum first, followed by the bile duct and the duodenum. An alternative for pancreatic-enteric reconstruction involves the use of a pancreaticogastrostomy. The biliary-enteric anastomosis is typically performed in end-to-side fashion approximately 10 cm down the jejunal limb from the pancreatic-enteric anastomosis. The third anastomosis is the duodenojejunostomy, typically performed 10 to 15 cm downstream from the biliary-enteric anastomosis.
The operative mortality rate for pancreaticoduodenectomy is currently less than 3% in major surgical centers with significant experience with the procedure. The leading causes of postoperative in-hospital death include postoperative sepsis, hemorrhage, and cardiovascular events. Although the mortality rate may be low, the incidence of postoperative complications can approach 40 to 50%. The leading causes of morbidity include early delayed gastric emptying and disruption or failure of healing of the pancreatic anastomosis with subsequent pancreatic fistula formation, as well as intra-abdominal abscess, hemorrhage, and wound infection (Table 3).
The prognosis for patients with resected adenocarcinoma of the head of the pancreas presents an overall 5-year survival of between 15 and 21%. The median postresection survival varies between 16 and 22 months. Factors determining overall prognosis include clinicopathologic staging, tumor biological features, molecular genetics, and the use of postoperative adjuvant therapy. Tumor characteristics are important predictors of survival include tumor diameter, lymph node status, and resection margin status.
TABLE 3. Complications of pancreaticoduodenectomy
Common |
Uncommon |
Delayed gastric emptying Pancreatic fistula Infra-abdominal abscess Hemorrhage Wound infection Metabolic Diabetes Pancreatic exocrine insufficiency |
Fistula Biliary Duodenal Gastric Organ failure Cardiac Hepatic Pulmonary Renal Pancreatitis Marginal ulceration |
Adenocarcinomas of the body and tail of the pancreas represent up to one third of all cases of pancreatic cancer. Tumors in this location generally grow to a large size before the development of symptoms. This silent course occurs as a result of the retroperitoneal location of the body and tail of the pancreas away from the common bile duct and duodenum. Thus, these tumors do not cause early obstructive jaundice or gastrointestinal obstructive symptoms. Concomitant with their large size, these tumors have a much higher incidence of metastatic disease at initial diagnosis. As a result, the likelihood that curative resection will be possible is low in most patients with such tumors. However, if the diagnosis is made when the tumor is localized and not encasing the celiac axis, the superior mesenteric vessels, or the portal vein, then resection remains a surgical option. In addition to routine staging studies including abdominal CT or MRI, staging laparoscopy appears to have an important role in patients with body and tail tumors.
At the time of exploration, a routine search for metastatic disease is performed. Additionally, the gastrocolic ligament should be opened to allow full assessment of the body and tail of the gland and evaluation of the tumor's proximity to the ligament of Treitz and to the superior mesenteric vessels. In the absence of findings of unresectability, mobilization of the inferior surface of the body of the pancreas from the retroperitoneum may be helpful to assess retroperitoneal involvement. Splenic preservation is not indicated when the resection is performed for pancreatic adenocarcinoma; therefore, the spleen is mobilized out of the retropehtoneum, often with early ligation of the splenic artery. Once the tumor has been elevated, the splenic vein can be controlled at a variable distance from its junction with the portal vein. The pancreatic parenchyma can then be divided, to leave a 1- to 2-cm margin of normal pancreas away from the tumor. Patients with the resection for adenocardnoma of the body and tail of the pancreas have median survival rates (from 7 to 13 months), with 5-year survival rates in the range of 10%. Such factors as tumor size, lymph node involvement, and surgical margins can all affect long-term survival. Data on the role of postoperative radiation or chemotherapy for these tumors are limited.
TESTS:
1. The commonest pancreatic tumour -
a) Ductal adenocarcinoma
b) Cystadenoma
c) Insulinoma
d) Non islet cell tumour
2. The commonest type of pancreatic carcinoma -
a) Acinar cell carcinoma
b) Ductal adenocarcinoma
c) Islet cell carcinoma
d) Mucinous carcinoma
3. Carcinoma of pancreas attains it’s greatest size when it is located in -
a) Head
b) Body and tail
c) Ampullary region
d) Ampula of vater
4. All are features of pesudopancreatic cyst, except -
a) Follows acute pancreatitis
b) Lined by false epithelium
c) May regress spontaneously
d) Treatment of choice is percutaneous aspiration
5. In Whipple's operation all structures are removed except -
a) CBD
b) Portal vein
c) Head of pancreas
d) Duodenum
6. Which of the following is the most common site for the carcinoma of pancreas -
a) head
b) Ampulla
c) Body
d) Neck
e) Tail
ГОУ ВПО «Смоленская государственная медицинская академия
Федерального агентства по здравоохранению и социальному развитию»
МЕТОДИЧЕСКИЕ УКАЗАНИЯ ДЛЯ СТУДЕНТОВ
ПО ДИСЦИПЛИНЕ хирургические болезни
OBSTRUCTIVE JAUNDICE, CHOLANGITIS (classification, differential diagnostics, surgical tactics). DRAINAGED AND RECONSTRUCTIVE OPERATIONS IN BILE SURGERY
Составитель асс. А.А.Безалтынных
Методические указания утверждены на методическом совещании кафедры госпитальной хирургии (протокол № 2 от 6 октября 2008 г.)
Зав. кафедрой______________(проф. С.А.Касумьян)
г.
THEME: OBSTRUCTIVE JAUNDICE
QUESTIONS FOR HOMEWORK:
1. Anatomy
2. Classification
3. Pathophysiology
4. Clinical symptoms.
5. Diagnostics.
6. Differential diagnosis.
7. Treatment.
8. Postoperative complications.
1.Introduction/Definition
Jaundice (derived from French word ‘jaune’ for yellow) or icterus (Latin word for jaundice) is a yellowish staining of the skin, sclera and mucous membranes by deposition of bilirubin (a yellow orange bile pigment) in these tissues. Jaundice was once called the "morbus regius" (the regal disease) in the belief that only the touch of a king could cure it. Jaundice indicates excessive levels of conjugated or unconjugated bilirubin in the blood and is clinically apparent when the bilirubin level exceeds 2mg/dl (34.2 µmol per L). It is most apparent in natural sunlight. In fact, it may be undetectable in artificial or poor light. In fair-skinned patients, jaundice is most noticeable on the face, trunk, and sclerae; in dark-skinned patients, it’s noticeable on the hard palate, sclerae, and conjunctivae. Pseudo jaundice may be found in black patients with pigmented sclera, from carotinemia, uremia (a sallow yellowish pallor), and quinacrine (a yellow-green color). Causes of jaundice can be classified into pre-hepatic, hepatic or post hepatic. In this review, our focus is on post hepatic causes of jaundice (obstructive or surgical cholestasis) as this is more relevant to surgeons. Obstructive jaundice is not a definitive diagnosis and early evaluation to establish the etiology of the cholestasis is crucial to avoid secondary pathological changes (e.g. secondary biliary cirrhosis) if obstruction is not relieved.
2. Surgical Anatomy of the Hepatobiliary system
An accurate knowledge of the anatomy of the liver and biliary tract, and their relationship to associated blood vessels is essential for the performance of hepatobiliary surgery because wide anatomic variations are common. The classic anatomic description of the biliary tract is only present in 58% of the population. The liver, gallbladder, and biliary tree arise as a ventral bud (hepatic diverticulum) from the most caudal part of the foregut early in the fourth week. This divides into two parts as it grows between the layers of the ventral mesentery: the larger cranial part (pars hepatica) is the primordium of the liver, and the smaller caudal part (pars cystica) expands to form the gallbladder, its stalk becoming the cystic duct. The initial connection between the hepatic diverticulum and the foregut narrows, thus forming the bile duct. As a result of the positional changes of the duodenum, the entrance of the bile duct is carried around to the dorsal aspect of the duodenum. The biliary system can be broadly divided into two components, the intra-hepatic and the extra-hepatic tracts. The secretory units of the liver (hepatocytes and biliary epithelial cells, including the peribiliary glands), the bile canaliculi, bile ductules (canals of Hering), and the intrahepatic bile ducts make up the intra-hepatic tract while the extra-hepatic bile ducts (right and left), the common hepatic duct, the cystic duct, the gallbladder, and the common bile duct constitute the extra-hepatic component of the biliary tree. The cystic and common hepatic ducts join to form the common bile duct. The common bile duct is approximately 8 to 10 cm in length and 0.4 to 0.8 cm in diameter. The common bile duct can be divided into three anatomic segments: supraduodenal, retroduodenal, and intrapancreatic. The common bile duct then enters the medial wall of the duodenum, courses tangentially through the submucosal layer for 1 to 2 cm, and terminates in the major papilla in the second portion of the duodenum. The distal portion of the duct is encircled by smooth muscle that forms the sphincter of Oddi. The common bile duct may enter the duodenum directly (25%) or join the pancreatic duct (75%) to form a common channel, termed the ampulla of Vater. The biliary tract is supplied by a complex vasculature called the peribiliary vascular plexus. Afferent vessels of this plexus derive from hepatic arterial branches, and this plexus drains into the portal venous system or directly into hepatic sinusoids.
3. Physiology/Biochemistry of Bilirubin production and transport
Bile is a substance produced in the liver and contains bile salts, water, cholesterol, electrolytes, and bilirubin, which is a breakdown product of hemoglobin. The formation of bilirubin from heme is essential for mammalian life, because it provides the body with the main means of elimination of heme. Eighty percent of the circulating bilirubin is derived from heme of hemoglobin from senescent red blood cells destroyed in the reticuloendothelium of the bone marrow, spleen, and liver. Ten to twenty percent of the bilirubin comes from other sources such as myoglobin, cytochromes, and other heme-containing proteins processed in the liver. Initially, heme is oxidized at the alpha position to the green pigment biliverdin, which is then reduced at the gamma position to bilirubin. Bilirubin is virtually insoluble in aqueous solutions. In blood it is reversibly but tightly bound to plasma albumin at a 1:1 ratio. Newly formed bilirubin is removed from the circulation very rapidly by the liver. The processing of the serum bilirubin load by the hepatocytes occurs in four steps. These are: uptake, cytosolic binding, conjugation, and secretion. Hepatic uptake of bilirubin occurs with the dissociation of the albumin-bilirubin complex facilitated by plasma membrane proteins with subsequent translocation of bilirubin into the hepatocyte through a saturable protein carrier, which also binds other organic anions, but not bile salts. In the hepatocytes, bilirubin binds to two cytosolic proteins: ligandin and Z protein. The binding limits the reflux of bilirubin back to the plasma and delivers it to the endoplasmic reticulum for conjugation. Conjugation of bilirubin involves its esterification with glucuronic acid to form, first, a monoglucuronide, then a diglucuronide. The principal enzyme involved is uridine diphosphate (UDP)-glucuronyl transferase. Conjugation renders bilirubin water-soluble and is essential for its elimination from the body in bile and urine. Most of the conjugated bilirubin excreted into bile in humans is diglucuronide with a lesser amount of monoglucuronide. Secretion of conjugated bilirubin from the hepatocyte to the bile canaliculi involves a specific carrier and occurs against a concentration gradient. Conjugated bilirubin is excreted in bile, as a micellar complex with cholesterol, phospholipids, and bile salts, through the biliary and cystic ducts to enter the gallbladder, where it is stored; or it passes through Vater’s ampulla to enter the duodenum. Inside the intestines, some bilirubin is excreted in the stool, while the rest is metabolized by the gut flora into urobilinogens and then reabsorbed. The majority of the urobilinogens are filtered from the blood by the kidney and excreted in the urine. A small percentage of the urobilinogens are reabsorbed in the intestines and re-excreted into the bile through the entero hepatic circulation. Recent findings in the field of molecular biology and the human genome project have highlighted various proteins and genes responsible for the metabolism of bilirubin and some of these are being exploited in the treatment of cholestasis.
4. Pathophysiology of obstructive jaundice
Bile is a multipurpose secretion with an array of functions, including intestinal digestion and absorption of lipids, elimination of environmental toxins, carcinogens, drugs, and their metabolites (xenobiotics), and serving as the primary route of excretion for a variety of endogenous compounds and metabolic products, such as cholesterol, bilirubin, and many hormones. In obstructive jaundice, the pathophysiologic effects reflect the absence of bile constituents (most importantly, bilirubin, bile salts, and lipids) in the intestines, and their backup, which causes spillage into the systemic circulation. Stools are often pale because less bilirubin reaches the intestine. Absence of bile salts can produce malabsorption, leading to steatorrhea and deficiencies of fat-soluble vitamins (particularly A, K, and D); vitamin K deficiency can reduce prothrombin levels. In long-standing cholestasis, concomitant vitamin D and Ca malabsorption can cause osteoporosis or osteomalacia. Bilirubin retention produces mixed hyperbilirubinemia. Some conjugated bilirubin reaches and darkens the urine. High levels of circulating bile salts are associated with, but may not cause, pruritus. Cholesterol and phospholipid retention produces hyperlipidemia despite fat malabsorption (although increased liver synthesis and decreased plasma esterification of cholesterol also contribute); triglyceride levels are largely unaffected. The lipids circulate as a unique, abnormal, low-density lipoprotein called lipoprotein X. Cholestatic liver diseases are characterized by accumulation of hepatotoxic substances, mitochondrial dysfunction and impairment of liver antioxidant defense. The storage of hydrophobic bile acids has been indicated as the main cause of hepatotoxicity with alteration of some important cell functions, such as the mitochondrial energy production. Both mitochondrial metabolism impairment and hydrophobic bile acids accumulation are associated with increased production of oxygen free radical species and development of oxidative damage.
5. Etiology
Myriad of diseases can lead to extra hepatic biliary obstruction The common ones include:
Choledocholithiasis
Cholangiocarcinoma,
Ampullary cancers,
Cancer of the Pancreas
Biliary strictures.
6. Clinical Features
A good history, physical examination and diagnostic tests are the requisites for the evaluation of the jaundiced patient. Jaundice, dark urine, pale stools and generalized pruritus are the hallmark of obstructive jaundice. History of fever, biliary colic and intermittent jaundice may be suggestive of cholangitis/choledocholithiasis. Weight loss, abdominal mass, pain radiating to the back and progressively deepening jaundice may be suggestive of pancreatic cancer. Deep jaundice (with a greenish hue) that appears to fluctuate in intensity may be due to a peri ampullary cancer. A palpably enlarged gall bladder in a jaundiced patient is also suggestive of an extrahepatic malignancy (Couvoissier’s statement).
7. Investigations
Biochemistry/Hematology Elevated serum bilirubin level with a preponderance of the conjugated fraction is the rule. The serum gamma glutamyl transpeptidase (GGT) level is also raised in cholestasis. In general, patients with gallstone disease have less hyperbilirubinemia than those with extra-hepatic malignant obstruction. The serum bilirubin is usually less than 20 mg/dL. The alkaline phosphatase may be elevated up to ten times normal. The transaminases may abruptly rise about ten times normal and decrease rapidly once the obstruction is relieved. Elevated WBC may be present in cholangitis. In pancreatic cancer and other obstructive cancers, the serum bilirubin may rise to 35 to 40 mg/dL, the alkaline phosphatase may rise up to ten times normal, but the transaminases may remain normal. Tumor markers like CA 19-9, CEA and CA-125 are usually elevated in pancreatic cancers, cholangiocarcinoma and peri-ampullary cancers, but they are non specific and may be elevated in other benign diseases of the hepatobiliary tree. Imaging the goals of imaging are: to confirm the presence of an extrahepatic obstruction (i.e., to verify that the jaundice is indeed post-hepatic rather than hepatic), to determine the level of the obstruction, to identify the specific cause of the obstruction, and to provide complementary information relating to the underlying diagnosis (e.g., staging information in cases of malignancy). A plain abdominal x ray may show calcified gallstones, porcelain gallbladder, air in the biliary tract or air in the gallbladder wall. Ultrasonography shows the size of the bile ducts, may define the level of the obstruction, may identify the cause and gives other information related to the disease (e.g. hepatic metastases, gallstones, hepatic parenchymal change). It identifies bile duct obstruction with 95% accuracy though results are largely operator dependent. It will also show stones in the gallbladder and dilated bile duct, but it is unreliable for small stones or strictures in the bile ducts. It may also demonstrate tumors, cysts, or abscesses in the pancreas, liver, and surrounding structures. In Africa, this is available in most centers and probably constitutes the main imaging modality available apart from X-ray. Computed tomography (CT) of the abdomen provides excellent visualization of the liver, gallbladder, pancreas, kidneys, and retroperitoneum. It can differentiate between intra- and extra-hepatic obstruction with 95% accuracy. However, CT may not define incomplete obstruction caused by small gallstones, tumors, or strictures. Contrast-enhanced multi-slice CT is very useful for assessment of biliary malignancies. Contrast agents given orally or intravenously are used and imaging done in unenhanced, arterial and venous phases. ERCP and PTC (Percutaneous transhepatic cholangiography) provide direct visualization of the level of obstruction. However they are invasive and associated with complications like cholangitis, biliary leakage, pancreatitis and bleeding. These facilities are generally not available in most centers in Africa. Endoscopic ultrasound: Endoscopic ultrasonography has various applications, such as staging of gastrointestinal malignancy, evaluation of submucosal tumors, and has grown to be an important modality in evaluating the pancreaticobiliary system. With regard to the biliary system, EUS is useful for the detection and staging of ampullary tumors, detection of microlithiasis, choledocholithiasis and evaluation of benign and malignant bile-duct strictures. It can further evaluate relationships to vascular structures. It may help define benign lesions mimicking cancer (e.g. sclerosing pancreatitis) if there is diagnostic doubt. Endoscopic ultrasound enables the aspiration of cysts and biopsy of solid lesions, but is operator-dependent. Unfortunately, this is not readily available in most centers in Africa. Magnetic resonance cholangiopancreatography (MRCP) is a newer, noninvasive technique for visualization of the biliary and pancreatic ductal system. It is especially useful in patients who have contraindications for endoscopic retrograde cholangiopancreatography (ERCP). Excellent visualization of biliary anatomy is possible without the invasiveness of ERCP. Unlike ERCP, it is purely diagnostic. Other imaging tests include Cholescintigraphy, radionuclide scanning (Tc 99) angiography and staging laparoscopy. These imaging facilities are hard to find in Africa and ultrasonography remains the only diagnostic test available in most centers.
8. Approach to the Jaundiced Patient
Barkun et al have written an excellent review on an approach to the jaundiced patient. I have summarized the approach with the following questions: Question 1: Is Jaundice present? A skin discoloration suggestive of jaundice can be mimicked by a variety of conditions which include: a) consumption of large quantities of food containing lycopene or carotene b) use of drugs like rifampicin or quinacrine It is therefore necessary to inspect not only the skin, but the mucous membranes of the mouth, palm, soles and the sclera. Q2: Is it Direct or Indirect Hyperbilirubinemia? Dark urine, pale stools and other features of cholestasis, like pruritus, are suggestive of direct hyperbilirubinemia, while normal colored urine and stool reflect unconjugated hyperbilirubinemia. In majority of cases, clinical findings alone will be sufficient to differentiate conjugated from unconjugated hyperbilirubinemia.
Q3: Is it Hepatic or Post hepatic? Once direct hyperbilirubinemia has been confirmed, the next question to answer is whether the jaundice is from hepatic or post-hepatic lesions. Clinical features of hepatic jaundice include history of alcohol abuse, acute hepatitis, and stigmata of chronic liver disease like palmar erythema, caput medusae, ascites and Dupuytren’s contracture. Post-hepatic jaundice usually present with abdominal pain, rigors, itching and palpable liver more than 2cm below the costal margin. Using clinical approach and simple biochemical tests (total serum bilirubin, alkaline phosphatase and gamma glutamyl transferrase levels) will usually give a good judgment on whether the jaundice is hepatic or post-hepatic. However, this approach will not be able to identify the level of the obstruction. Q4: What is the level of the obstruction? Imaging is the key to identifying the level of obstruction. Ultrasonography will be able to identify the level of obstruction in about 90% of cases. Other imaging facilities like MRCP, ERCP, PTC, and CT scan may be used where Ultrasonography can not determine the level of the obstruction. Q5: What is the cause of the obstruction? The commonest cause of obstruction in the West is usually choledocholithiasis. However, if choledocholithiasis is excluded, pancreatic and peri ampullary cancers are the next common causes. Q6. What is the extent of the disease (staging)/complications (cholangitis)? While obvious metastases may be present by a palpation of a nodular enlarged liver or other evidence of widespread disease, sophisticated imaging is required for more precise staging. Fever and elevated WBC are indicative of cholangitis. Q7. If it is malignant, is it respectable? Assessment of the resectability of a tumor usually hinges on whether the superior mesenteric vein, the portal vein, the superior mesenteric artery, and the porta hepatis are free of tumor and on whether there is evidence of significant local adenopathy or extrapancreatic extension of tumor. Multislice spiral CT is the imaging of choice for assessment of respectability of pancreatic cancers. Optimal evaluation is achieved with a fine-cut dual-phase (arterial phase and portal venous phase). MRCP, EUS, CT angiography or duplex Doppler Ultrasonography are other imaging facilities that can be used in assessment of hepatobiliary malignancies in centers where they are available. For unresectable malignancies, the choice is between surgical palliation/bypass and ERCP/PTC with drainage. In some cases, neither option may be feasible because of advanced disease; in such a case supportive care alone will suffice. For lesions that are respectable or amenable to surgical palliation, the choice of treatment will depend on the level of obstruction and the precise etiology. For this purpose, the lesions can be classified into three:
a) Upper third obstruction: Surgical palliation is best achieved with a left (segment 3) hepaticojejunostomy (The long extrahepatic course of the left hepatic duct makes it more accessible). For respectable lesions, the tumor is resected with a possible hepatectomy or segmentectomy and reconstruction achieved by hepaticojejunostomy or cholangiojejunostomy.
b) Middle third obstruction: Surgical palliation is easier and hepaticojejunostomy after the bifurcation is done. If tumor is resectable, reconstruction is achieved with hepaticojejunostomy.
c) Lower third obstruction: Surgical palliation done using a Roux en Y choledochojejunostomy. Cholecystojejunostomy carries a high risk of complications and subsequent jaundice. If tumor is respectable, a pancreatiduodenectomy (Whipple’s procedure) or local ampullary resection should be done.
9. Treatment
Extrahepatic biliary obstruction requires mechanical decompression. Other goals include treatment of the underlying cause, symptoms, and complications (e.g., vitamin malabsorption). Decompression of extrahepatic biliary obstruction can be achieved by any of these three methods: surgical bypass, resection of obstructing lesions, percutaneous insertion of stents, and endoscopic insertion of stents.
9.1. General Considerations
Pruritus usually subsides with correction of the underlying disorder or with 2 to 8 gm. orally of cholestyramine bid, which binds bile salts in the intestine. However, this is ineffective in complete biliary obstruction. Unless severe hepatocellular damage is present, hypoprothrombinemia usually subsides after use of (vitamin K1) 5 to 10 mg sc once/day for 2 to 3 days. Ca and vitamin D supplements, with or without a bisphosphonate, slow the progression of osteoporosis only slightly in long-standing irreversible cholestasis. Vitamin A supplements prevent deficiency and severe steatorrhea can be minimized by replacing some dietary fat with medium-chain triglycerides. Jaundiced patients undergoing surgery for large bile duct obstruction (from any cause) are subject to specific risks that require prophylactic measures. These include:
infections (cholangitis, septicaemia, wound infections )
bleeding (non-coagulant acarboxyl derivatives of vitamin K dependent factors)
renal failure
liver failure
fluid and electrolyte abnormalities
Preparation for surgery is important because of the associated perioperative morbidity previously discussed. The specific measures required in all patients are:
parenteral administration of vitamin K analogues – to normalise prothrombin time
intravenous hydration and catheterization of the urinary bladder
forced natriuresis by mannitol with induction of anaesthesia
antibiotic prophylaxis against gram negative aerobes – using a three-dose regimen
frozen section should be booked for all patients undergoing resection for cancer
9.2. Specific Treatment based on causes
9.2.1. Choledocholithiasis (bile duct stones)
There are various options available. The best option should be individualized and based on the following factors:
Physical condition of the patient including co morbidity and medical history
Previous attempts at intervention or previous cholecystectomy
Availability of equipment/theatre/anesthetist/expertise of Interventionist
Patient preference.
Open exploration of the common bile duct: involves:
Cholecystectomy, if present.
supraduodenal longitudinal choledochotomy
Extraction of calculi by Fogarty balloon trawl, Desjardins forceps or Dormia basket and irrigation with saline.
Confirmation of duct clearance superiorly and inferiorly by choledochoscopy and/or cholangiography.
Where facilities for choledochoscopy and intraoperative cholangiogram are not available, to avoid the risk of leaving retained duct stones, a T tube is usually inserted to confirm clearance of the duct by a postoperative cholangiogram after at least five days. The T tube is removed after two weeks, when an epithelialzed tract has formed to avoid bile leak into the peritoneal cavity. Several trials however have shown that primary closure of the bile duct without T tube is as safe as using T tube and is associated with less complications like sepsis, tube migrations and bile peritonitis. In Africa and other developing countries where there may be no facilities for intraoperative cholangiogram or intraoperative Ultrasonography, T tube placement will be a pragmatic approach. Unfortunately, in most centers, T tubes are hard to find. Other procedures in difficult cases: Removal of common bile duct calculi may prove difficult by any of the above methods, for example:
impacted stone when all efforts to remove it have failed
multiple large stones
inaccessible duct (e.g. previous surgery, unfit patient).
Surgical or percutaneous drainage procedures may be useful. Choledochoduodenostomy may be done by anastomosis of a dilated common bile duct to the duodenum. Alternatively, particularly in a non-dilated duct, a transduodenal sphincteroplasty is undertaken by first carrying out an open sphincterotomy and stone extraction, then suturing the mucosa of the duct and duodenum together to keep the lower end patent; these procedures are rarely undertaken. Percutaneous stenting or naso-biliary drainage may be done in an unfit patient with common bile duct stones that cannot be removed by ERCP ERCP±sphincterotomy: A cholangiogram is done after the ampulla of Vater has been identified and cannulated to confirm anatomy and the presence of stones. An adequate sphincterotomy is undertaken and the duct cleared using a balloon catheter or Dormia basket. Confirmation of duct clearance should be established with a radiograph. If the stones are too large, they can be crushed in situ using a mechanical lithotripter; however care should be exercised to avoid damage to the duct lining. Other techniques described in the literature include extracorporeal shockwave lithotripsy, contact lithotripsy, laser under direct vision. These are however time consuming, resource intensive and are limited to few specialized centers. Endoscopic placement of a stent, or temporary naso-biliary drainage can be a good option if the stones are multiple or too large for extraction. This relieves obstruction and prevents impaction of stones at the ampulla of Vater. Success rate after ERCP±sphincterotomy is about 90% with low complications in experienced hands. Complications include perforation, acute pancreatitis, and bleeding from damage to a branch of the superior pancreatico-duodenal artery. Difficulties may arise as a result of technical problems in cannulating the ampulla of Vater or anatomical anomalies like duodenal diverticulum ERCP may be considered the definitive treatment for some unfit patients, but most will proceed to cholecystectomy to remove remaining gallstones and prevent further complications. Endoscopic balloon dilation was introduced about three decades ago for elderly and frail patients as an alternative to sphincterotomy, because of the advantages of preserving the sphincter of Oddi. This has been abandoned in North America because of the risk of pancreatitis. It is still practiced in parts of Asia and Europe. A recent Cochrane review concluded that it is slightly less successful than endoscopic sphincterotomy in stone extraction and more risky regarding pancreatitis and probably has a clinical role in patients who have coagulopathy, who are at risk for infection, and possibly in those who are older. Laparoscopic exploration of the common bile duct may be done through the cystic duct (if the gall bladder has not been previously removed) or common duct via a choledochotomy. Stones are extracted under fluoroscopic guidance using balloon catheters or Dormia basket. Choledochoscopy and lithotripsy can also be done for larger stones. This technique requires considerable laparoscopic expertise and is time consuming, so it is rarely the first-line treatment for common bile duct stones; these are usually removed at ERCP preoperatively and a laparoscopic cholecystectomy done electively. Single stage laparoscopic cholecystectomy and ductal stone clearance has been shown in several studies to have the same efficacy and morbidity with the staged approach with the added benefit of reduced costs. Nevertheless, most centers still favor preoperative endoscopic ductal clearance because LECBD is technically demanding and sophisticated laparoscopic equipment may not be available in every surgical unit. Medical dissolution of common bile duct stones: Flushing with normal saline; infusion of bile salts, monooctanoin, methyl tert-butyl ether, or other solvents into the CBD through a Ttube are medical remedies for choledocholithiasis that have been described in the literature. The efficacy of the surgical/endoscopic approaches to bile duct stones have made medical approaches unattractive. The principal disadvantages of bile acid infusion are the prolonged period of hospitalization required to carry out the treatment, the unsatisfactory handling of distal occluding stones and those on the hepatic side of the T-tube, the high incidence of side effects, and the rather unpredictable outcome.
9.2.2. Cholangiocarcinoma
Cholangiocarcinomas are epithelial cancers of the cholangiocytes and they can occur at any level of the biliary tree. They are broadly classified into intra-hepatic tumours, (extra-hepatic) hilar tumours and (extra-hepatic) distal bile duct tumours. Majority arise in the absence of risk factors, however identified risk factors include age, primary sclerosing cholangitis, chronic choledocholithiasis, bile duct adenoma, biliary papillomatosis, Caroli’s disease, choledochal cyst, thorotrast, smoking, parasitic biliary infestation and chronic typhoid carrier state. Hilar cholangiocarcinoma accounts for two thirds of all cases of extra-hepatic cholangiocarcinoma. Intra-hepatic and distal extra-hepatic cholangiocarcinomas are less common, but surgical resection remains the only chance of cure consisting of liver resection and pancreaticoduodenectomy, respectively. Unfortunately, the majority of these tumors are unresectable, Surgery is the only curative option for cholangiocarcinoma. The extent of spread, available surgical expertise and associated co-morbidities are important factors that will determine the treatment approach. Although several surgical series have been reported, recent trends are to advocate accurate preoperative staging with an aggressive onco-surgical approach involving en-bloc hilar or hepatic resections. Currently, cholecystectomy, lobar or extended lobar hepatic and bile duct resection, regional lymphadenectomy, and Roux-en-Y hepaticojejunostomy are the treatments of choice for hilar cholangiocarcinoma. Encouraging reports with the use of photofrin based photodynamic therapy have been reported in the literature. Systemic therapy/Palliative therapy: The majority of patients with cholangiocarcinoma present at an advanced stage or have associated co-morbidity that preclude surgery. For these patients, the goal of treatment is to obtain adequate palliation. Biliary endoprosthesis (stent) placement is a useful option for palliation of jaundice. The approach is usually by ERCP but for proximal lesions the transhepatic route may be used. Photodynamic therapy, radiation and chemotherapy are all available as palliative options. Several chemotherapeutic agents have been evaluated with limited results. Gemcitabine or 5-Fluorouracil are the two common agents used as a single agent or in combination with other drugs.
9.2.3. Ampullary tumours
Peri-ampullary cancers can be broadly considered as tumors arising within 1 cm of the ampulla of Vater and include ampullary, distal bile duct, pancreatic, and duodenal cancers. However, without careful histological analysis, it is difficult if not impossible to differentiate the tumor type. Surgical excision is the mainstay of treatment for peri-ampullary cancers. Careful preoperative staging and assessment of respectability is crucial. If the tumor is resectable, the procedure of choice is a pancreaticoduodenectomy. The classical approach (Whipple’s procedure or cWhipple) described by Kausch and Whipple remains the most popular technique in North America and Europe. The more conservative approach (pylorus preserving Whipple resection or ppWhipple) described by Watson in 1943 and later popularized by Traverso and Longmire is another technique that is gradually gaining more converts. Pylorus-preserving pancreaticoduodenectomy is reported to be an easier and less time-consuming operation with less blood loss, a shorter hospital stay, and better weight gain during follow-up care. Also, no differences in the recurrence rate and patient survival exist between pylorus-preserving pancreaticoduodenectomy and the standard Whipple procedure. For unresectable tumors, palliative treatment will depend on comorbidity factors, and availability of resources and expertise for endoscopic treatment. Biliary bypass procedures can be done operatively, laparoscopically, endoscopic stenting or by percutaneous transhepatic approaches. Gastric bypass procedures may also be indicated in patients with gastric outlet obstruction. The role of prophylactic gastric bypass procedures is controversial, however a prospective randomized clinical trial concluded that a prophylactic gastrojejunostomy significantly decreases the incidence of late gastric outlet obstruction and did not increase the incidence of postoperative complications or extend the length of stay.
9.2.4. Pancreatic Cancer
Pancreatic ductal adenocarcinoma is one the most lethal GI malignancy with an overall 5-year survival rate of less than 4%. Factors influencing this grim prognosis are 1) clinical symptoms in the early stage are usually absent or non specific resulting in late diagnosis, with only 15–20% of tumors being respectable at presentation. 2) Clinically, aggressive growth, with retroperitoneal and perineural infiltration, angioinvasion, high rates of local relapse, formation of metastases, and 3) resistance to most of the available treatment regimens, makes patient management a complex and challenging task. The only hope for cure is surgery, but unfortunately less than 20% are respectable. There is now an acceptable operative mortality rate of less than or equal to 5% for resected patients when performed at experienced or dedicated centers with high volume of patients in the western world. The treatment options are similar to peri-ampullary cancers. The role of adjuvant therapy in advanced pancreatic cancer is controversial as most of the trials show limited benefits. Gemcitabine, 5FU are agents that show some promise. Pain Palliation: Patients who present with severe pain must receive opioids. Morphine is generally the drug of choice. Usually, the oral route is preferred in routine practice. Parenteral routes of administration should be considered for patients who have impaired swallowing or gastrointestinal obstruction. Percutaneous celiac plexus blockade can be considered, especially for patients who experience poor tolerance of opiate analgesics.
9.2.5. Biliary Strictures
Biliary strictures can be benign or malignant. In this section of the review, our focus will be on benign biliary strictures as the common causes of malignant strictures have been treated earlier. The majority of benign strictures are iatrogenic - as a result of operations on the gallbladder and the biliary tree. The introduction of laparoscopic cholecystectomy initially led to an increase in operative trauma to the bile ducts from 0.1-0.2% to 2%. This was not surprising considering the steep learning curve of laparoscopic procedures. However, after widespread adoption of lap cholecystectomy, the incidence of operative trauma still remains higher than what obtained in the era of open cholecystectomy at 0.2-0.7%. Non iatrogenic causes of benign strictures include inflammatory conditions and subsequent fibrosis related to chronic pancreatitis, cholelithiasis, choledocholithiasis, sclerosing cholangitis, stenosis of the sphincter of Oddi, or infections of the biliary tract. Three options for the management of benign biliary strictures are currently available: percutaneous dilation and stenting, endoscopic dilation and stenting, and surgical biliary drainage, most commonly by a Roux-en-Y hepaticojejunostomy. All the options have comparable results, with stricture relapse rates reported between 15%–45% and mean follow-up times of 4–9 years. The choice of treatment modality must be individualized and should be based on the following considerations: the location and severity of the stricture, the presence of biliary-enteric continuity, the degree of infection, over-all health of the individual patient, the length of time anticipated for stenting, and the need for repeated dilation and stent exchange. It calls for a close collaboration between the surgeon and the interventional radiologist.
10. Complications
Complications of obstructive jaundice include sepsis especially cholangitis, biliary cirrhosis, pancreatitis, coagulopathy, renal and liver failure. Other complications are related to the underlying disease and the procedures employed in the diagnosis and management of individual diseases. Cholangitis especially the suppurative type (Charcot’s triad or Raynaud’s pentad) is usually secondary to choledocholithiasis. It may also complicate procedures like ERCP. Treatment should include correction of coagulopathy, fluid/electrolyte anomaly, antibiotics and biliary drainage with ERCP where available or trans-hepatic drainage or surgery.
11. Conclusion
Obstructive jaundice is a clinical diagnosis that requires both clinical and diagnostic work up to elucidate the precise etiology. A multi disciplinary approach that requires the clinician, radiologist, endoscopist and interventional radiologist will lead to a better outcome.
12. Recommendations
Treatment should be individualized - based on patient factors and availability of resources and personnel.
To optimize treatment for pancreatic cancers, dedicated centers should be established.
Pylorus preserving resection is recommended instead of the Classical Whipple’s resection.
Extensive palliative procedures carry a significant degree of morbidity and mortality in advanced hepatobiliary malignancies and should be discouraged.
Need for training in endoscopic procedures for African surgeons.
ERCP is preferred to trans-hepatic drainage for biliary decompression except for obstructions near the hepatic bifurcation.
Primary closure of the common bile duct after exploration for stones is as safe as leaving a T tube in situ and associated with fewer complications if confirmation of biliary clearance can be obtained.
FURTHER READING
1. Roche SP, Kobos R. Jaundice in the adult patient.[see comment]. [Review] [20 refs]. American Family Physician 69(2):299-304, 2004.
2. C D BriggsM Peterson. Investigation and management of obstructive jaundice. Surgery 25[2], 74-80. 2007.
3. Koenraad J.Mortelé and Pablo R.Ros. Anatomic Variants of the Biliary Tree MR Cholangiographic Findings and Clinical Applications . AJR Am.J.Roentgenol. 177, 389-394. 2001.
4. M.LAMAH 1NDKAGHD. Anatomical Variations of the Extrahepatic Biliary Tree: Review of the World Literature. Clinical Anatomy 14, 167-172. 2001. http://simplelink.library.utoronto.ca/url.cfm/27873
5. Roche SP, Kobos R. Jaundice in the adult patient.[see comment]. [Review] [20 refs]. American Family Physician 69(2):299-304, 2004. http://simplelink.library.utoronto.ca/url.cfm/27868
6. Boyer JL. New perspectives for the treatment of cholestasis: lessons from basic science applied clinically. [Review] [79 refs]. Journal of Hepatology 46(3):365-71, 2007. http://simplelink.library.utoronto.ca/url.cfm/27878
7. Malhi H, Gores GJ, Malhi H, Gores GJ. Review article: the modern diagnosis and therapy of cholangiocarcinoma. [Review] [78 refs]. Alimentary Pharmacology & Therapeutics 2006; 23(9):1287-1296.
8. Yusuf TE, Bhutani MS, Yusuf TE, Bhutani MS. Role of endoscopic ultrasonography in diseases of the extrahepatic biliary system. [Review] [45 refs]. Journal of Gastroenterology & Hepatology 2004; 19(3):243-250. http://simplelink.library.utoronto.ca/url.cfm/27882
9. Baron TH. Palliation of malignant obstructive jaundice. Gastroenterol Clin North Am 2006; 35(1):101-112.
THEME: CHOLANGITIS
QUESTIONS FOR HOMEWORK:
1. Anatomy
2. Classification
3. Pathophysiology
4. Clinical symptoms.
5. Diagnostics.
6. Differential diagnosis.
7. Treatment.
8. Postoperative complications.
INTRODUCTION
Background: Acute cholangitis is a bacterial infection superimposed on an obstruction of the biliary tree most commonly from a gallstone, but it may be associated with neoplasm or stricture.
Pathophysiology: The main factors in the pathogenesis of acute cholangitis are biliary tract obstruction, elevated intraluminal pressure, and infection of bile. It is believed that biliary obstruction diminishes host antibacterial defenses, causes immune dysfunction, and subsequently increases small bowel bacterial colonization. Although the exact mechanism is unclear, it is believed that bacteria gain access to the biliary tree by retrograde ascent from the duodenum or from portal venous blood. As a result, infection ascends into the hepatic ducts, causing serious infection. Increased biliary pressure pushes the infection into the biliary canaliculi, hepatic veins, and perihepatic lymphatics, leading to bacteremia (25-40%). The infection can be suppurative in the biliary tract.
The bile is normally sterile. In the presence of gallbladder or common duct stones (CBD), however, the, incidence of bactibilia increases. The most common organisms cultured in cholangitis are Escherichia coli (39%), Klebsiella (54%) and Enterobacter (34%) species, enterococci (34%), and group D streptococci. The infection may also be polymicrobial. For related pathophysiology, please see the Cholelithiasis and Cholecystitis and Biliary Colic articles.
Frequency:
In the US: Cholangitis is relatively uncommon. It occurs in association with other diseases that cause biliary obstruction and bactibilia (eg, after endoscopic retrograde cholangiopancreatography [ERCP], 1-3% of patients develop cholangitis). Risk is increased if dye is injected retrograde.
Internationally: Oriental cholangiohepatitis is endemic in Southeast Asia. This is a recurrent pyogenic cholangitis with intrahepatic and extrahepatic stones in 70-80% of patients and cholelithiasis in 50-70%.
Mortality/Morbidity:
Mortality of cholangitis is high due to the predisposition in people with underlying disease. Historically, mortality was 100%. Currently, mortality ranges from 7-40%.
The following patient characteristics are associated with a higher morbidity and mortality:
Hypotension
Acute renal failure
Liver abscess
Cirrhosis
Inflammatory bowel disease
High malignant strictures
Radiologic cholangitis – Post percutaneous transhepatic cholangiography
Female gender
Age older than 50 years
Failure to respond to antibiotics and conservative therapy
Advanced age, concurrent medical problems, and delay in decompression increase emergent operative mortality (17-40%). Mortality of elective surgery after medical stabilization is significantly less (approximately 3%). In the past, suppurative cholangitis was thought to have increased morbidity; however, prospective studies have not found this to be true.
Race:
Cholangitis is frequently associated with gallstones and risk factors are the same. Prevalence of gallstones is highest in fair-skinned people of Northern European descent as well as Hispanic populations, Native Americans, and Pima Indians.
In addition, certain Asian populations and inhabitants of countries where intestinal parasites are common are also at increased risk. Asians are more likely to have primary stones due to chronic biliary infections, parasites, bile stasis, and biliary strictures. Recurrent pyogenic cholangitis (oriental cholangiohepatitis) rarely is observed in the US but may be observed in Asian populations. Although it tends to be less severe, the ducts and liver are damaged.
African Americans are only at increased risk if they have a hematologic disorder (eg, sickle cell anemia).
Sex:
While gallstones are more common in women, the male-to-female ratio is equal in cholangitis.
Age:
Elderly patients are more likely to progress from asymptomatic gallstones to serious complications of gallstones and cholangitis without gallbladder colic.
Suspect cholangitis in older patients presenting with sepsis and mental status changes. Elderly patients are more prone to gallstones and CBD stones and, therefore, cholangitis.
The median age at presentation is between 50 and 60 years.
CLINICAL
History: In 1877, Charcot described cholangitis as a triad of findings of right upper quadrant (RUQ) pain, fever, and jaundice. The Reynolds pentad adds mental status changes and sepsis to the triad. A spectrum of cholangitis exists, ranging from mild symptoms to fulminant overwhelming sepsis. With septic shock, diagnosis can be missed in up to 25% of patients. Consider cholangitis in any patient who appears septic, especially with patients who are elderly, jaundiced, or who have abdominal pain. History of abdominal pain or past symptoms of gallbladder colic helps make the diagnosis.
Charcot triad of fever, RUQ pain, and jaundice is found in 50-70% of patients presenting with cholangitis.
Fever is present in approximately 90% of cases. Abdominal pain and jaundice is thought to occur in 70% and 60% of patients, respectively.
Patients present with altered mental status 10-20% of the time and hypotension approximately 30% of the time. These signs combined with Charcot triad constitute Reynolds pentad.
Most patients complain of RUQ pain; however, some patients (ie, elderly persons) are too ill to localize the source of infection.
Other symptoms include the following:
Jaundice
Fever, chills, and rigors
Pruritus
Acholic or hypocholic stools
The patient's past medical history may be helpful. For example, a history of the following increases the risk of cholangitis:
Gallstones, CBD stones
Recent cholecystectomy
Endoscopic manipulation or ERCP, cholangiogram
History of cholangitis
History of HIV or AIDS: AIDS-related cholangitis is characterized by extrahepatic biliary edema, ulceration, and obstruction. Etiology is uncertain but may be related to cytomegalovirus or cryptosporidium infections. Manage cholangitis as described below, although decompression usually is not necessary.
Physical:
Patients with cholangitis generally are quite ill and frequently present in septic shock without an apparent source.
Physical examination may reveal the following:
Fever (90%), although elderly patients may have no fever
RUQ tenderness (65%)
Mild hepatomegaly
Jaundice (60%)
Mental status changes (10-20%)
Sepsis
Hypotension (30%)
Tachycardia
Peritonitis (unusual; should lead to a search for an alternative diagnosis)
Causes:
Choledocholithiasis is the most common cause of acute cholangitis, followed by ERCP and tumors.
Stasis or obstruction of bile in the CBD leads to bacterial infection and cholangitis. Partial obstruction is associated with a higher rate of infection than complete obstruction.
CBD stones predispose patients to cholangitis.
Approximately 10-15% of patients with cholecystitis have CBD stones.
Approximately 1% of patients post-cholecystectomy have retained CBD stones. Most CBD stones are immediately symptomatic, while some remain asymptomatic for years.
Some CBD stones are formed primarily rather than secondary to gallstones.
Obstructive tumors cause cholangitis. Partial obstruction is associated with an increased rate of infection compared with that of complete neoplastic obstruction.
Pancreatic cancer
Cholangiocarcinoma
Ampullary cancer
Porta hepatis tumors or metastasis
Additional causes of cholangitis include the following:
Strictures or stenosis
Endoscopic manipulation of the CBD
Choledochocele
Sclerosing cholangitis (from biliary sclerosis)
AIDS cholangiopathy
Ascaris lumbricoides infections
DIFFERENTIALS
Cholecystitis and Biliary Colic
Diverticular Disease
Hepatitis
Mesenteric Ischemia
Pancreatitis
Shock, Septic
Other Problems to be Considered:
Cirrhosis Liver failure
Liver abscess
Acute appendicitis
Perforated peptic ulcer
Pyelonephrosis Right colon diverticulitis
WORKUP
Lab Studies:
CBC: Leukocytosis: In patients with cholangitis, 79% had a WBC greater than 10,000, with a mean of 13.6. Septic patients may be leukopenic.
Comprehensive metabolic panel with bicarbonate
Expect liver function test results to be consistent with cholestasis, hyperbilirubinemia (88-100%), and increased alkaline phosphatase (78%).
Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels are usually mildly elevated.
Check renal function and electrolytes as well. Calcium is necessary if concern from pancreatitis exists.
Prothrombin time and activated partial thromboplastin time: Do not expect either to be elevated unless sepsis is associated with disseminated intravascular coagulation or underlying cirrhosis exists. A coagulation profile may be required if the patient needs operative intervention.
Blood cultures (2 sets): Between 20 and 30% of blood cultures are positive. Many exhibit polymicrobial infections.
Urinalysis (usually normal)
Blood type, screen, and crossmatch: With urgent operating room dispatch, patients need to have blood available.
Amylase and/or lipase: Involvement of the lower CBD may cause elevated amylase and frank pancreatitis. One third of patients have mildly elevated amylase.
Biliary cultures (not an ED procedure): Send biliary cultures if the patient has biliary drainage by interventional radiology or endoscopy.
Imaging Studies:
Imaging studies are important to confirm the presence and cause of biliary obstruction and to rule out other conditions. Ultrasonography and CT scanning are the most commonly used first-line imaging modalities.
Endoscopic retrograde cholangiopancreatography (ERCP) is both diagnostic and therapeutic and is considered the criterion standard for imaging the biliary system.
ERCP should be reserved for patients who may require therapeutic intervention.
Diagnostic use of ERCP carries a complication rate of approximately 1.38% and a mortality rate of 0.21%. The major complication rate of therapeutic ERCP is 5.4% and a mortality rate of 0.49%.
In general, abdominal films aid little in the diagnosis of acute cholangitis.
An ileus may be observed.
Between 10 and 30% of gallstones have a ring of calcium and as a result are radiopaque.
Patients may show air in the biliary tree after endoscopic manipulation or if they have emphysematous cholecystitis, cholangitis, or a cholecystic-enteric fistula.
Air in the gallbladder wall indicates emphysematous cholecystitis.
Ultrasound is excellent for gallstones and cholecystitis and is fairly sensitive for intrahepatic and extrahepatic dilation, including CBD dilation; however, it is not as useful in detecting choledocholithiasis.
Ultrasound can differentiate intrahepatic from extrahepatic obstruction and image dilated ducts.
In one study of cholangitis, only 13% of CBD stones were observed on ultrasound, but dilated CBD was found in 64%.
Advantages to sonography include the ability to be performed rapidly at the bedside by the ED physician, capacity to image other structures (eg, aorta, pancreas, liver), identification of complications (eg, perforation, empyema, abscess), and lack of radiation.
Disadvantages to sonography include operator and patient dependence, no possible imaging of the cystic duct, and decreased sensitivity for CBD stones.
A normal sonogram does not rule out acute cholangitis.
CT scan is adjunctive to and may replace ultrasound. Spiral or helical CT scan improves imaging of the biliary tree. A CT cholangiography uses a contrast agent that is taken up by the hepatocytes and secreted into the biliary system. This enhances the ability to visualize radiolucent stones and increases detection of other biliary pathology.
Dilated intrahepatic and extrahepatic ducts and inflammation of the biliary tree are imaged.
Gallstones are poorly visualized with traditional CT scan.
Advantages of CT scan include the following:
Other pathologies that are causes or complications of cholangitis (eg, ampullary tumors, pericholecystic fluid, liver abscesses) can be imaged.
Pathology that must be distinguished from cholangitis also can be observed (eg, right-sided diverticulitis, papillary necrosis, some evidence of pyelonephrosis or mesenteric ischemia, ruptured appendix).
Detection of biliary pathology with CT cholangiography approaches that of ERCP.
Disadvantages of CT scan include poor imaging of gallstones, allergic reaction to contrast, and diminished ability to visualize the biliary tree with elevated serum bilirubin.
Magnetic resonance cholangiopancreatography (MRCP) is a noninvasive imaging modality that is increasingly being used in the diagnosis of biliary stones and other biliary pathology.
MRCP is accurate for detecting choledocholithiasis, neoplasms, strictures, and dilations within the biliary system.
Limitations of MRCP include the inability for invasive diagnostic tests such as bile sampling, cytologic testing, stone removal, or stenting.
Absolute contraindications are the same for a traditional MRI, which include the presence of a cardiac pacemaker, cerebral aneurysm clips, ocular or cochlear implants, and ocular foreign bodies. Relative contraindications include the presence of cardiac prosthetic valves, neurostimulators, metal prostheses, and penile implants.
The risk of MRCP during pregnancy is not known.
Other Tests:
Biliary scintigraphy (hepatic 2,6-dimethyliminodiacetic acid [HIDA] and diisopropyl iminodiacetic acid [DISIDA])
HIDA and DISIDA scans are functional studies of the gallbladder.
Obstruction of the CBD causes nonvisualization of the small intestine. A HIDA scan with complete biliary obstruction does not visualize the biliary tree.
Advantages include its ability to assess function, and positive results may appear before the ducts are enlarged sonographically.
One disadvantage is that high bilirubin levels (>4.4) may decrease the sensitivity of the study. Recent eating or no food in 24 hours also may affect the study. In addition, anatomic imaging for other structures is lacking. The study takes several hours, so it may be ill advised for unstable patients.
Procedures:
ED physicians generally do not perform procedures for cholangitis, ERCP, and transhepatic decompression.
If an obstruction is observed, ERCP provides direct visualization and potential treatment. It is best performed after 72 hours of antibiotics or after resolution of fever.
In unstable patients, a reasonable option for decompression of the biliary tract is percutaneous transhepatic cholangiogram and biliary drain. The biliary ducts are observed, even when no ductal dilatation is present.
TREATMENT
Prehospital Care:
Diagnosis of cholangitis is not a prehospital diagnosis. Mild cholangitis may present with abdominal pain, jaundice, and fever. When transporting these patients to the hospital, place the patient on a monitor and insert an intravenous (IV) line.
In unstable patients with cholangitis, prehospital care should include the following:
Immediate assessment of ABCs
Monitoring (eg, pulse oximetry, cardiac monitor, frequent blood pressure measurements, blood glucose measurement)
Stabilization (eg, oxygen, placement of 2 large-bore IVs, administration of IV fluids to unstable patients)
Rapid transport
Emergency Department Care:
Suspect mild cholangitis in patients with jaundice and a fever; consider cholangitis in all patients with sepsis.
Degree of urgency of treatment depends on severity of illness. Important points are resuscitation, diagnosis, and treatment.
After assessment of the ABCs, place the patient on a monitor with pulse oximetry, provide oxygen via nasal canula, and obtain an ECG. Draw and send labs (including blood cultures) when the IV is placed.
Administer parenteral antibiotics empirically after blood cultures are drawn. Do not delay administration of antibiotics if blood cultures cannot be drawn.
For management of patients in septic shock.
Standard therapy for cholangitis consists of broad-spectrum antibiotics with close observation to determine the need for emergency decompression of the biliary tree.
A nasogastric tube may be helpful for patients with vomiting.
Patient should be nothing by mouth (NPO). Place a Foley catheter in ill patients to monitor urine output.
The surgical literature states that, in patients with mild cholangitis, 70-85% respond to medical therapy. Approximately 15% do not respond and subsequently require immediate surgical or endoscopic decompression.
Mortality rates approach 100% for patients who fail medical therapy and do not have surgical decompression.
In severely ill patients, treatment is immediate biliary decompression. The method depends on the degree of illness. In the past, drainage was performed surgically. Today, options of percutaneous or endoscopic drainage exist in addition to medical management with antibiotics. Endoscopic drainage has been shown to decrease mortality from 30% to 10%.
Medical therapy can be complementary to surgical or endoscopic treatments. In less ill patients, medical treatment may be all that is necessary. Most of the literature recommends medical therapy with IV antibiotics for 12-24 hours.
Maintain medical therapy and consider elective surgery with patients who show improvement. Refer patients who deteriorate to ERCP and sphincterotomy or percutaneous drainage.
Consultations:
Immediately consult surgery and gastroenterology.
While most patients respond to antibiotics and conservative care, a subset requires emergent procedures. In deciding to drain, consult with gastroenterology and surgery.
Increased mortality is observed in patients with hypotension, acute renal failure, liver abscess, cirrhosis, high malignant strictures, female gender, and advanced age. Therefore, consider these patients earlier for decompression. Patients with malignant obstruction usually do not respond to antibiotics (59% compared to 85%).
Unstable septic patients require clinical judgment to determine if they will survive until medical therapy has a chance to work or if they require emergency decompression with its associated high mortality.
MEDICATION
Debate exists as to whether the most effective antibiotics must have high biliary concentrations. When high intrabiliary pressures exist due to biliary obstruction, whether any antibiotic is excreted effectively into the bile is doubtful, thus making biliary levels irrelevant. The choice of antibiotics should be guided by local sensitivity patterns.
Traditional therapy with ampicillin and an aminoglycoside is now a less ideal regimen secondary to weakened activity of ampicillin against both aerobic and anaerobic gram-negative bacilli, and concern for nephrotoxicity of aminoglycosides.
Many newer combinations have been shown to be effective as either a single agent or combination therapy. Combinations include extended-spectrum cephalosporin, metronidazole, and ampicillin. Single agent regimens include piperacillin and tazobactam; mezlocillin; imipenem; meropenem; ticarcillin and clavulanate; or ampicillin and sulbactam, which can also be combined with metronidazole.
The following dosages are general recommendations. Please check current sources prior to administration.
Drug Category: Antibiotics -- The antibiotic regimen must cover enteric microbes, including the most common organisms: E coli (39%), Klebsiella (54%) and Enterobacter (34%) species, enterococci (34%), and group D streptococci.
Drug Name |
Ampicillin (Omnipen, Marcillin) -- Interferes with bacterial cell wall synthesis during active multiplication, causing bactericidal activity against susceptible organisms. Must be used in combination. |
Adult Dose |
2 g IV q6h |
Pediatric Dose |
50 mg/kg IV q6h |
Contraindications |
Documented hypersensitivity |
Interactions |
Probenecid and disulfiram elevate ampicillin levels; allopurinol decreases ampicillin effects and has additive effects on ampicillin rash; may decrease effects of oral contraceptives |
Pregnancy |
B - Usually safe but benefits must outweigh the risks. |
Precautions |
Adjust dose in renal failure; evaluate rash and differentiate from hypersensitivity reaction |
Drug Name |
Piperacillin (Pipracil) -- Inhibits biosynthesis of cell wall mucopeptide and is effective during stage of active multiplication. Has antipseudomonal activity. |
Adult Dose |
4 g IV q6h |
Pediatric Dose |
Not established |
Contraindications |
Documented hypersensitivity |
Interactions |
Tetracyclines may decrease effects; piperacillin at high concentrations may physically inactivate aminoglycosides; probenecid may increase levels of piperacillin; coadministration with aminoglycosides has synergistic effects |
Pregnancy |
B - Usually safe but benefits must outweigh the risks. |
Precautions |
Caution in renal impairment and in history of seizures |
Drug Name |
Metronidazole (Flagyl) -- Imidazole ring-based antibiotic active against various anaerobic bacteria and protozoa. Usually employed in combination with other antimicrobial agents. |
Adult Dose |
1 g IV loading dose, followed by 500 mg IV q6h or 1 g IV q12h |
Pediatric Dose |
7.5-15 mg/kg/d IV divided bid |
Contraindications |
Documented hypersensitivity |
Interactions |
May increase toxicity of anticoagulants, lithium, and phenytoin; cimetidine may increase toxicity of metronidazole; disulfiram reaction may occur with orally ingested ethanol |
Pregnancy |
B - Usually safe but benefits must outweigh the risks. |
Precautions |
Adjust dose in hepatic disease; monitor for seizures and development of peripheral neuropathy |
Drug Name
|
Gentamicin (Gentacidin, Garamycin) -- Aminoglycoside antibiotic for gram-negative coverage. Used in combination with both an agent against gram-positive organisms and one that covers anaerobes. Not DOC. Consider if penicillins or other less toxic drugs are contraindicated, when clinically indicated, and in mixed infections caused by susceptible staphylococci and gram-negative organisms. Dosing regimens are numerous; adjust dose based on CrCl and changes in volume of distribution. May be given IV/IM. Follow each regimen by at least a trough level drawn on the third or fourth dose (0.5 h before dosing); may draw a peak level 0.5 h after 30-min infusion. |
Adult Dose |
3-5 mg/kg/d IV divided tid |
Pediatric Dose |
5-7 mg/kg/d IV divided tid |
Contraindications |
Documented hypersensitivity; nondialysis-dependent renal insufficiency |
Interactions |
Coadministration with other aminoglycosides, cephalosporins, penicillins, and amphotericin B may increase nephrotoxicity; aminoglycosides enhance effects of neuromuscular blocking agents, thus prolonged respiratory depression may occur Coadministration with loop diuretics may increase auditory toxicity of aminoglycosides; possible irreversible hearing loss of varying degrees may occur (monitor regularly) |
Pregnancy |
D - Unsafe in pregnancy |
Precautions |
Monitor gentamicin levels to prevent ototoxicity; narrow therapeutic index (not intended for long-term therapy); caution in patients with renal failure who are not on dialysis, myasthenia gravis, hypocalcemia, and conditions that depress neuromuscular transmission; adjust dose in renal impairment |
Drug Name |
Piperacillin/tazobactam (Zosyn) -- Antipseudomonal penicillin plus beta-lactamase inhibitor. Inhibits biosynthesis of cell wall mucopeptide and is effective during stage of active multiplication. Used in combination therapy. |
Adult Dose |
3.375 g IV q6h |
Pediatric Dose |
75 mg/kg IV q6h |
Contraindications |
Documented hypersensitivity; do not treat severe pneumonia, bacteremia, pericarditis, emphysema, meningitis, and purulent or septic arthritis with oral penicillin during acute stage |
Interactions |
Tetracyclines may decrease effects of ticarcillin; high concentrations of ticarcillin may physically inactivate aminoglycosides if administered in same IV line; effects when administered concurrently with aminoglycosides are synergistic; probenecid may increase penicillin levels |
Pregnancy |
B - Usually safe but benefits must outweigh the risks. |
Precautions |
Tetracyclines may decrease effects of ticarcillin; high concentrations of ticarcillin may physically inactivate aminoglycosides if administered in same IV line; effects when administered concurrently with aminoglycosides are synergistic; probenecid may increase penicillin levels |
Drug Name |
Cefotaxime (Claforan) -- Third-generation cephalosporin that has broad gram-negative spectrum, lower efficacy against gram-positive organisms, and higher efficacy against resistant organisms. Arrests bacterial cell wall synthesis and inhibits bacterial growth by binding to one or more of the penicillin-binding proteins. Can be used in combination with metronidazole or clindamycin. |
Adult Dose |
1 g IV q8-12h |
Pediatric Dose |
80-180 mg/kg/d IV divided tid/qid |
Contraindications |
Documented hypersensitivity |
Interactions |
Probenecid may increase cefotaxime levels; coadministration with furosemide and aminoglycosides may increase nephrotoxicity |
Pregnancy |
B - Usually safe but benefits must outweigh the risks. |
Precautions |
Adjust dose in severe renal impairment; has been associated with severe colitis |
Drug Name |
Clindamycin (Cleocin) -- Lincosamide for treatment of serious skin and soft tissue staphylococcal infections. Also effective against aerobic and anaerobic streptococci (except enterococci). Inhibits bacterial growth, possibly by blocking dissociation of peptidyl tRNA from ribosomes, causing RNA-dependent protein synthesis to arrest. |
Adult Dose |
600 mg IV q6-8h |
Pediatric Dose |
15-40 mg/kg IV divided tid/qid |
Contraindications |
Documented hypersensitivity; regional enteritis; ulcerative colitis; hepatic impairment; antibiotic-associated colitis |
Interactions |
Increases duration of neuromuscular blockade induced by tubocurarine and pancuronium; erythromycin may antagonize effects of clindamycin; antidiarrheals may delay absorption of clindamycin |
Pregnancy |
B - Usually safe but benefits must outweigh the risks. |
Precautions |
Adjust dose in severe hepatic dysfunction; no adjustment necessary in renal insufficiency; associated with severe and possibly fatal colitis |
Drug Name |
Mezlocillin (Mezlin) -- During growth phase, interferes with bacterial cell wall synthesis, causing death in susceptible microorganisms. Has antipseudomonal activity. Use in combination therapies. |
Adult Dose |
3 g IV q4h |
Pediatric Dose |
300 mg/kg/d IV/IM divided q4-6h; not to exceed 24 g/d |
Contraindications |
Documented hypersensitivity |
Interactions |
Administered concomitantly with aminoglycosides, has synergistic effects; probenecid increases mezlocillin blood levels; administered concurrently with vecuronium, duration of neuromuscular blockade increases; enhances anticoagulant effects of heparin; may decrease effectiveness of oral contraceptives; bacteriostatic effects of tetracyclines may decrease effectiveness of penicillins |
Pregnancy |
B - Usually safe but benefits must outweigh the risks. |
Precautions |
Caution in preexisting sinus node dysfunction and renal impairment, bradycardias, antiarrhythmic agents, thrombocytopenia, electrolyte disturbances, or congestive heart failure |
Drug Name |
Imipenem and cilastatin (Primaxin) -- A carbapenem; may be used alone or in combination. Used for treatment of multiple-organism infections for which other agents do not have wide-spectrum coverage or are contraindicated due to potential for toxicity. |
Adult Dose |
0.5 g IV q6h |
Pediatric Dose |
<12 years: Not established; 15-25 mg/kg/dose IV q6h suggested for >3 mo Fully susceptible organisms: Not to exceed 2 g/d Moderately susceptible organisms: Not to exceed 4 g/d >12 years: Administer as in adults |
Contraindications |
Documented hypersensitivity |
Interactions |
Coadministration with cyclosporine may increase CNS adverse effects of both agents; coadministration with ganciclovir may result in generalized seizures |
Pregnancy |
C - Safety for use during pregnancy has not been established. |
Precautions |
Adjust dose in renal insufficiency; avoid use in children <12 y |
Drug Name |
Meropenem (Merrem) -- A carbapenem; may be used alone or in combination. Broad-spectrum carbapenem antibiotic that inhibits cell-wall synthesis and has bactericidal activity. Effective against most gram-positive and gram-negative bacteria. Has slightly increased activity against gram-negative organisms and slightly decreased activity against staphylococci and streptococci compared to imipenem. |
Adult Dose |
0.5-1 g IV q6h |
Pediatric Dose |
40 mg/kg IV q8h |
Contraindications |
Documented hypersensitivity |
Interactions |
Probenecid may inhibit renal excretion of meropenem, increasing meropenem levels |
Pregnancy |
B - Usually safe but benefits must outweigh the risks. |
Precautions |
Pseudomembranous colitis and thrombocytopenia may occur, requiring immediate discontinuation of medication |
Drug Name |
Ticarcillin and clavulanate potassium (Timentin) -- Inhibits biosynthesis of cell wall mucopeptide and is effective during stage of active growth. Antipseudomonal penicillin plus a beta-lactamase inhibitor that provides coverage against most gram-positive and gram-negative organisms and most anaerobes. |
Adult Dose |
3.1 g IV q4-6h |
Pediatric Dose |
75 mg/kg IV q6h |
Contraindications |
Documented hypersensitivity; severe pneumonia, bacteremia, pericarditis, emphysema, meningitis, and purulent or septic arthritis should not be treated with oral penicillin during acute stage |
Interactions |
Tetracyclines may decrease effects of ticarcillin; high concentrations of ticarcillin may physically inactivate aminoglycosides if administered in same IV line; effects when administered concurrently with aminoglycosides are synergistic; probenecid may increase penicillin levels |
Pregnancy |
B - Usually safe but benefits must outweigh the risks. |
Precautions |
Perform CBCs prior to initiation of therapy and at least weekly during therapy; monitor for liver function abnormalities by measuring AST and ALT during therapy; exercise caution in patients diagnosed with hepatic insufficiencies; perform urinalysis and BUN and creatinine determinations during therapy and adjust dose if values become elevated; monitor blood levels to avoid possible neurotoxic reactions |
Drug Name |
Ampicillin and sulbactam sodium (Unasyn) -- Combination antimicrobial agent that uses a beta-lactamase inhibitor with ampicillin. Covers skin, enteric flora, and anaerobes. Not ideal for nosocomial pathogens. |
Adult Dose |
1.5 (1 g ampicillin + 0.5 g sulbactam) to 3 g (2 g ampicillin + 1 g sulbactam) IV/IM q6-8h; not to exceed 4 g/d sulbactam or 8 g/d ampicillin |
Pediatric Dose |
3 months to 12 years: 100-200 mg ampicillin/kg/d (150-300 mg Unasyn) IV divided q6h >12 years: Administer as in adults; not to exceed 4 g/d sulbactam or 8 g/d ampicillin |
Contraindications |
Documented hypersensitivity |
Interactions |
Probenecid and disulfiram elevate ampicillin levels; allopurinol decreases ampicillin effects and has additive effects on ampicillin rash; may decrease effects of oral contraceptives |
Pregnancy |
B - Usually safe but benefits must outweigh the risks. |
Precautions |
Adjust dose in renal failure; evaluate rash and differentiate from hypersensitivity reaction |
FOLLOW-UP
Further Inpatient Care:
Admission to ICU for ill patients is appropriate.
Continue IV antibiotics.
Monitor blood cultures and narrow spectrum of antibiotics as appropriate.
Administer IV antibiotics 12-24 hours.
Refer worsening patients to emergent ERCP for sphincterotomy or percutaneous drainage.
Cholecystectomy or ERCP is best after resolution of the cholangitis.
Transfer:
Transfer is appropriate in hospitals unable to manage significantly ill patients with intensive medical care and surgery and endoscopic consultation.
Optimize patient stabilization prior to transfer.
Minimum initial stabilization includes the following:
Appropriate diagnostics
ABCs (including volume resuscitation)
Administration of broad-spectrum antibiotics
Critical care transport
Deterrence/Prevention:
Prophylactic antibiotics prior to ERCP may decrease risk of cholangitis.
Prompt recognition and treatment of symptomatic cholelithiasis in patients at higher risk for complications (eg, those with diabetes) decrease risk of cholangitis.
Aggressive search for CBD stones during diagnosis and treatment of cholecystitis may be necessary to prevent cholangitis.
Complications:
Patients are increasingly likely to have complications with greater degrees of illness, as follows:
Liver failure, hepatic abscesses, and microabscesses
Bacteremia (25-40%); gram-negative sepsis
Acute renal failure
Catheter-related problems in patients treated with percutaneous or endoscopic drainage
Bleeding (intra-abdominally or percutaneously)
Catheter-related sepsis
Fistulae
Bile leak (intraperitoneally or percutaneously)
Prognosis:
Prognosis depends on several factors.
Early recognition and treatment of cholangitis
Response to therapy
Underlying medical conditions of the patient
Mortality ranges from 7-40%, with much higher mortality in patients who require emergency decompression or surgery.
In patients responding to antibiotic therapy, the prognosis is good.
MISCELLANEOUS
Special Concerns:
Since pregnant women are prone to symptomatic gallstones, consider cholangitis in pregnant, febrile, or jaundiced patients. Differentiate cholangitis from HELLP syndrome (hemolysis, elevated liver enzymes, low platelet count) of preeclampsia, which also can cause abdominal pain and elevated LFTs. Blood pressure is elevated in preeclampsia and may be hypotensive in cholangitis.
Cholelithiasis and cholangitis are uncommon in children, except those with underlying hemolytic disorders or biliary anomalies.
The incidence of cholangitis is higher in elderly persons, most likely due to the increased prevalence of common bile duct stones with age. As in other infections and abdominal processes, elderly patients frequently do not manifest pathology in a classic pattern. Consider cholangitis in febrile or hypotensive elderly patients.
FURTHER READING
Bornman PC, van Beljon JI, Krige JE: Management of cholangitis. J Hepatobiliary Pancreat Surg 2003; 10(6): 406-14
Hanau LH, Steigbigel NH: Acute (ascending) cholangitis. Infect Dis Clin North Am 2000 Sep; 14(3): 521-46.
Muir CA: Acute ascending cholangitis. Clin J Oncol Nurs 2004 Apr; 8(2): 157-60.
Romagnuolo J, Bardou M, Rahme E, et al: Magnetic resonance cholangiopancreatography: a meta-analysis of test performance in suspected biliary disease. Ann Intern Med 2003 Oct 7; 139(7): 547-57.
TESTS
1. Most common route of infection/cause, for pyogenic liver abcess is -
a) Biliary sepsis
b) Stone
c) Appendicitis
d) Ca colon
2. A gall stone gets impacted most commonly in which part of common bile duct -
a) Supraduodenal
b) Retroduodenal
c) Ampulla of vater
d) Common hepatic duct
3. Common bile duct stones will manifest all except -
a) Distended gall bladder
b) Jaundice
c) Itching
d) Clay coloured stools
4. The following can be done in obstructive jaundice -
a) Vitamin K injections
b) Vitamin C injections
c) Dehydration therapy
d) External drainage
5. In cholangitis, the organism mostly responsible is -
a) E. coli
b) Streptococcus
c) E. Histolytica
d) Clostridium
6. Triad of Jaundice, chills and fever occurs in -
a) CBD stones
b) Post cholecystectomy
c) Acute hepatitis
d) Pancreatitis
7. Best diagnostic tool in obstructive jaundice is -
a) ERCP
b) Ultrasound
c) PTC
d) Blood tests
8. Which of the following is the commonest site for impaction of the gall stones -
a) Common hepatic duct
b) Ampulla of vater
c) Retroduodenal portion of common bile duct
d) Supradodenal portion of common bile duct
9. An ultrasound examination shows dilated intrahepatic biliary channels with a small gall baldder. The most likely possibility is -
a) Gall bladder stone
b) Pancreatic calculus
c) Common bile duct stone
d) Carcinoma of the head of the pancreas
10. Best suture for common bile duct is -
a) Synthetic absorbable synthetic
b) Synthetic non-absorbable
c) Non-synthetic absorbable
d) Non-synthetic non-absorbable
11. Klatskin tumour is tumour -
a) At the juncture of cystic duct and bile duct
b) At bile duct
c) At ampulla of vater
d) At the junction of the bile duct
12. Whenever there is stone in the bile duct which off the following raises -
a) Bile salts
b) Bilirubin
c) Amylase
d) SGPT
13. Most common cause of benign surgical jaundice is
a) Stricture
b) Atresia of extrahepatic ducts
c) Stone in CBD
d) Parasitic infestation of bileary tract
14. Charot's triad is seen in -
a) Acute pancreatitis
b) Hemobilia
c) Stone in bile duct
d) Chronic cholecystitis
15. After exploration of common bile duct, the T-Tube is removed on which of the following days -
a) 3 postop.day
b) 4 postop.day
c) 12 postop.day
d) 6 postop.day
16. An ultrasound examination shows dilated intrahepatic biliary channels with a small gall bladder. The most likely possibility is -
a) Gall baldder stone .
b) Pancreatic calculus
c) Common bile duct stone
d) Carcinoma of the head of the pancreas
17. The most common cause of suppurative cholangitis is-
a) Stone in common bile duct
b) Cancer of the ampulla of Vater
c) Choledochal cyst
d) Empyema of gall bladder
18. Investigation of choice of case of obstructive jaundice
a) USG
b) Plain X-ray
c) CT scan
d) ERCP
19. After surgery, a small left over stone in the CBD is best treated by -
a) ERCP
b) ESWL
c) Heparinised saline through T tube
d) Repeat surgery
20. Gold standard investigation for stone in bile-duct is
a) USG
b) ERCP
c) Cholangiography
d) CT scan
e) MRI
21. Treatment of choice of a CBD stone of 2.5 cms size, diagnosed 2 years after cholecystectomy -
a) Choledochotomy and T tube
b) Dormia basket
c) Transduodenal sphincterotomy
d) Supraduodenal choledochotomy
22. Bleeding in a case of obstructive jaundice is treated with -
a) Fresh frozen plasma
b) Cryoprecipitate
c) Whole blood
d) Buffy coat extract
Answers
A
C
A
A, D
A
A
A
B
C
C
D
B
C
C
None
C
A
A
A
B
C
A
ГОУ ВПО «Смоленская государственная медицинская академия
Федерального агентства по здравоохранению и социальному развитию»
МЕТОДИЧЕСКИЕ УКАЗАНИЯ ДЛЯ СТУДЕНТОВ
ПО ДИСЦИПЛИНЕ хирургические болезни
COMPLICATIONS OF GASTRIC AND DUODENAL ULCERS
Составитель доц. Ю.Я.Чайковский
Методические указания утверждены на методическом совещании кафедры госпитальной хирургии (протокол № 2 от 6 октября 2008 г.)
Зав. кафедрой______________(проф. С.А.Касумьян)
2008 г.
The student should know:
Anatomico-physiological features of a stomach and a duodenum;
Phases and regulating mechanisms of gastric secretion;
Modern views on an etiology and a pathogenesis of a peptic ulcer,
The pathoanathomical changes arising in a stomach and a duodenum at a peptic
ulcer and its complications;
A clinical presentation of an uncomplicated peptic ulcer,
A clinical presentation of the major complications: bleedings, a stenosis, perforation, a penetration and a malignancy;
Special methods of inspection of patients with a peptic ulcer;
Absolute and relative indications to operative treatment of a peptic ulcer;
Surgical tactics at the complicated ulcer.
Indication to surgery.
1. Absolute sings involve: perforation, profuse bleeding;
2. Next indication: stenosis malignant or ulcer-cancer single or multiple bleedings, calculous and penetration gastric ulcers;
3. Sings: mimic or obscure perforating, calculous and penetrating duodenal ulcers;
4. Relative signs may be: inability or inadequacy of conservative (medication) therapies for 2-3 years.
Peptic ulcer treatment involves a great many drugs
Antacids;
Sedatives which influence CNS activities;
H-2 receptor histaminblockers (e.g. Cimetidine, Quamatel, Ranitidine); M-cholinoblockers ( non-selective - Atropine, Metacine, Platifillin; and selective -Gastroreserpine);
Proton-pump inhibitors (PPI) - Omeprazole, Losec;
Preventive medicines (gastroprotectors for membrane - Almagel, Fosphalugel, De-Nol, Tribimol, Cytotec, Doksa, Misoprostol;
Antimicrobials - e.g. Metronidazol, De-Nol.
PERFORATED PEPTIC ULCER
Sex. The ratio is 2 men to 1 woman.
Age. The highest incidence is between 45 and 55 years.
Most often a peptic ulcer that perforates is situated on the anterior surface of the duodenum; much less frequently it is situated on the anterior surface of the stomach, usually near the lesser curvature or the pyloric antrum. Rarely an ulcer on the posterior wall of the stomach perforates into the lesser sac. In 80 per cent of cases, there is a history - often a long history -of peptic ulceration. In 20 per cent there is no such history; it is a 'silent' chronic ulcer that perforates especially in those patients who are being treated with cortisone. Usually, the symptoms of perforation occur with dramatic suddenness.
The gastric or duodenal contents escape through the perforation into the general peritoneal cavity, resulting in peritoneal irritation (peritonism). At that moment the victim cries out in agony and, at any rate if the perforation is a large one and the stomach is full, he or she is riveted temporarily to the spot where the perforation felled him or her. The peritoneum reacts to this chemical irritation by secreting peritoneal fluid copiously and this gives relief of pain for a short time. This stage of reaction lasts from 3 to 6 hours, and is followed by diffuse bacterial peritonitis.
Clinical features. Massive perforation. In the early stage of peritoneal irritation, the patient is pale, anxious and loath to move. The temperature may be subnormal but the pulse is raised. The abdomen is held still, moving little or not at all with respiration (Fig. 1). The whole abdomen is tender with board-like rigidity. It is dull to percussion. Sufficient gas may have escaped to reduce liver dullness in the midaxill-ary line. Pelvic tenderness may be elicited on rectal examination.
Fig. 1. A sketch of Mr Hamilton Bailey watching
for abdominal movement on respiration.
In cases of perforated peptic ulcer abdominal movement is restricted or absent.
After 3-6 hours, the pain, tenderness and rigidity may lessen. The temperature rises to normal or higher. However, the pulse remains high, the bowel sounds are absent. This temporary improvement has rightly been called the 'period of illusion'.
After 6 hours, the stage of diffuse peritonitis develops, accompanied by silent abdominal distension. Enough free fluid may have collected to be clinically detectable. The rising pulse rate marks the progressive deterioration in the patient's condition with each hour that passes without operative treatment.
Slow perforation. The pain may be less severe, less generalised with definite tenderness but equivocal guarding and rigidity when the perforation is small, and occurs in a robust, phlegmatic patient. Bowel sounds frequently persist. A small amount of fluid may track down the paracolic gutter, producing pain and tenderness in the right iliac fossa, simulating appendicitis. It is important to establish the site of onset of the pain.
D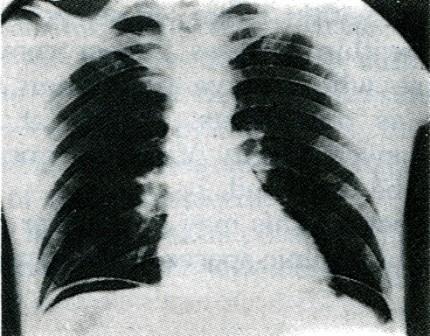 iagnostic
aids. A
plain radiograph of the abdomen with the patient erect reveals a
translucent area beneath the right cupola of the diaphragm in 70 per
cent of cases (Fig. 2). Bile-stained fluid may be aspirated from the
peritoneal cavity; it is alkaline to litmus.
iagnostic
aids. A
plain radiograph of the abdomen with the patient erect reveals a
translucent area beneath the right cupola of the diaphragm in 70 per
cent of cases (Fig. 2). Bile-stained fluid may be aspirated from the
peritoneal cavity; it is alkaline to litmus.
Fig. 2. Plain radiograph of a perforated
duodenal ulcer showing gas beneath the diaphragm.
(Dr R. Vecht, Bristol.)
Treatment. Morphine should not be given until written permission for operation has been obtained. Operation, as soon as the general condition permits, is usually the best course. Laparotomy is performed and the typical oedema with a clean punched-out ulcer of the anterior wall of the juxtapyloric region is found. The perforation is closed with interrupted sutures reinforced with an omental patch. In the case of a gastric ulcer a biopsy must be taken as there is a risk of malignancy. In large perforations it is advisable to reinforce the suture line with a patch of omentum.. Thorough peritoneal toilet is essential. With a sucker, fluid and food debris are removed from the peritoneal cavity. Drainage of the peritoneal cavity is employed.
The immediate after-treatment consists of continuous gastric aspiration supplemented by intravenous fluid. Antibiotic therapy and breathing exercises are important in elderly people, and, in cases where operation is more than 6 hours after perforation.
Endoscopic closure of perforation. A new era of minimally invasive surgery is beginning. It is now possible to inspect the peritoneal cavity, previously inflated with carbon dioxide, by means of a colour TV camera attached to a telescope inserted through a 10 mm diameter access port. By using a needle holder inserted through a second port and forceps via a third, the perforation may be closed. The peritoneal cavity may then be washed out and the patient spared an abdominal incision. A minimal leak on an empty stomach can be treated expectantly by continuous gastric aspiration.
Pyloroplasty with vagotomy may be carried out only if the patient would merit elective surgery for the ulcer before the perforation occurred, if the perforation is recent, if the patient is fit, and if the surgeon is experienced and backed by a fully trained supporting team (Kirk).
Follow-up of patients after perforation. As might be expected, there is a transient remission of symptoms due to the rest in bed and the careful dietetic supervision during convalescence. Elderly patients and those of any age with a short dyspeptic history are likely to remain symptom-free after successful treatment of a perforation (Illingworth).
Nevertheless, within 1 year, 40 per cent of patients relapse, and within 5 years 70 per cent. On this account, those who survive perforation should be followed up as outpatients, so that if symptoms suggesting renewed activity of the ulcer occur, timely treatment can be instituted.
Residual abscess. This may occur after perforation in any of the subphrenic spaces or in the pelvis.
CONSERVATIVE TREATMENT OF PERFORATED ULCER (TAYLOR TECHNIQUE)
- Bed regimen (Fouler position);
- Cold application on epigastria region;
- Continuous gastric aspiration on ("brushing");
- Antacids (anti - acid medication);
- Antibiotics / antimicrobials;
- Paraenteral feeding / meal;
- Post - syndrome and symptomatic treatment
TESTS
1. The factors of peritoneal inflammation are as follows:
1. Direct transition of inflammation from the organs onto the serous membrane.
2. Abdominal penetrating wound.
3. Injury of abdominal parenchymal organ.
4. Dehiscence of suture of anastomosis of hollow organs.
5. Ulcerous perforation of the intestinal wall.
Choose the right combination of answers:
A. 2, 3,4. B. 2, 3,4, 5. С. 1, 2, 4, 5. D. 3, 4, 5. E. 2, 3,4,5.
2. The specific pathogenic factors of peritonitis are as follows:
1. Spreading of the process.
2. GI paralysis.
3. Toxic dysfunction of the viscera.
4. Absorption of bacterial toxins by the peritoneum.
5. Absorption of bacterial toxins from the intestines.
Choose the right combination of answers:
A. 1, 3, 4, 5. B. 1, 2, 3, 4, 5. С. 1, 3, 4. D. 1, 3, 5. E.2, 3,5.
3. Diffuse peritonitis is diagnosed if the inflammation affects:
A. One area.
B. Three areas.
C. Four areas.
D. More than five areas. Choose the correct answer.
4. The signs of generalized suppurative peritonitis are as follows:
1. Bradycardia.
2. Abdominal muscle guarding.
3. Gaseous abdominal distension.
4. Fluid accumulation in sloping areas of the abdominal cavity.
5. Continual fever.
Choose the right combination of answers:
A. 1, 3, 4. B. 2, 4, 5. С. 2, 3, 4, 5. D. 2, 4, 5.
5. The clinical stages of peritonitis are as follows:
1. Paralysis.
2. Toxicity.
3. Generalized.
4. Multiple organ failure.
5. Transude.
6. Reactive.
Choose the right combination of answers:
A. 1,4, 6. B. 2, 4, 6. С. 2,4, 5. D. 1,2, 3. E. 2, 3, 4.
6. The clinical signs of toxic phase of peritonitis are as follows:
1. Abdominal distension.
2. Hypotension.
3. Tachycardia.
4. Vomiting and thirst.
5. Dyspnoea.
6. Absence of intestinal peristaltic sounds.
Choose the right combination of answers:
A. 1, 2, 4, 5. B. 2, 3, 4, 5. С. 1, 2, 5, 6. D. 2, 4, 5, 6. E. 1,2,3,4, 5,6.
7. The risk factors of generalized peritonitis that preclude urgent surgery are as follows:
1. Recent myocardial infarction.
2. Severe traumatic shock with concomitant injury.
3. Agony.
4. Post-operative peritonitis.
5. No risk factors.
Choose the right combination of answers:
A. 1,2, 3. B. 1,3,4. С. 2,3. D. 1,3. E. 5.
8. The treatment of generalized peritonitis involves the following measures:
1. Elimination of the causative agent.
2. Abdominal cleansing and drainage.
3. Correction of fluid and electrolyte imbalance.
4. Antibacterial therapy.
5. Detoxication therapy.
Choose the right combination of answers:
A. 1,3, 5. B. 3, 4, 5. С. 3,4. D. 2, 3, 4. E. 1, 2,3,4,5.
9. Commonest site of peptic ulcer perforation -
a) Anterior aspect of the first part of duodenum
b) Posterior aspect of the 1st part of duodenum
c) Greater cuvature of the stomach
d) Lesser curvature of the stomach
e) Anterior apect of 2nd of duodenum
10. The most essential feature in reaching a diagnosis of hypertrophic pyloric stenosis is –
a) Visible peristalsis
b) Projectile vomiting
c) Loss of weight and dehydration
d) Palpation of the hypertrophic pylorus
11. Percentage of patient with perforated peptic ulcer who show free gas under the diaphragm -
a) 100%
b) 75%
c) 50%
d) 90%
12. In pyloric stenosis the following changes occur-
a) Hypokalemic hyponatremic alkalosis
b) Hyperkalaemia
c) Acidosis with hyponartremic alkalosis
d) Hyperchloremic acidosis
13. Commonest operation done for peptic ulcer with gastric outlet obstruction is
Truncal vagotomy with pyloroplasty
Highly selective vagotomy with pyloroplasty
Truncal vagotomy with gastrojejunostomy
Gastrojejunostomy
14. The treatment of peptic ulcer involves -
Antacids
Ranitidine
Sucralfate
All
15. Malignant transformation is commonly seen in -
Stomach ulcer
Gastric ulcer
Chronic Duodenal ulcer
Post bulbar ulcer
16. The treatment of choice of hypertropnic pyloric stenosis of adults is –
a) Pyloromytomy
b) Pyloroplasty
c) Billroth I gastrectomy
d) Highly selective vagotomy
17. Investigation of choice in peptic ulcer perforation is
a) USG
b) X-Ray abdomen
c) Paracentasis
d) CTscan
18. What complication commonly occurs in anterior duodenal ulcer –
a) Bleeding
b) Penetration
c) Perforation
d) Stricture formation
ANSWERS:
1 - C
2 - B
3 - D
4 - C
5 - B
6 - E
7 - C
8 - E
9 - A
10 - D
11 - B
12 - A
13 - C
14 - D
15 - B
16 - B
17 - B
18 - C
ГОУ ВПО «Смоленская государственная медицинская академия
Федерального агентства по здравоохранению и социальному развитию»
МЕТОДИЧЕСКИЕ УКАЗАНИЯ ДЛЯ СТУДЕНТОВ
ПО ДИСЦИПЛИНЕ хирургические болезни
GASTRODUODENAL BLEEDING (classification, diagnostics, surgical tactics)
Составитель доц. Ю.И.Ломаченко
Методические указания утверждены на методическом совещании кафедры госпитальной хирургии (протокол № 2 от 6 октября 2008 г.)
Зав. кафедрой______________(проф. С.А.Касумьян)
2008 г.
UNIT: SURGICAL GASTROENTEROLOGY
Theme: ACUTE UPPER GASTROINTESTINAL BLEEDING
KEY QUESTIONS FOR HOMEWORK:
Causes of hemorrhage of the upper gastrointestinal tract.
Clinical signs.
Profuse gastrointestinal bleeding.
Endoscopical treatment.
Clinical approach to the patient's management.
Bleeding gastric and duodenal ulcers.
Mallory-Weiss Syndrome.
Hemorrhagic erosive gastritis.
Gastro-oesophageal variceal haemorrhage.
Arterio-venous malformation (Dieulafoy's disease).
Gastric carcinoma.
Prognosis.
Gastrointestinal bleeding in older people.
Causes of bleeding are many, and some of the more important ones are listed below:
Hemorrhagic esophagitis
Esophageal varices
Cancer of the esophagus
Mallory-Weiss syndrome
Erosive gastritis
Gastric ulcer
Stomach cancer
Duodenal ulcer
Arterio-venous malformation (Dieulafoy's disease)
Gastric or duodenal polyps
Acid-peptic disease (including ulcers, erosions, and gastritis) as a group accounts for the majority (50-75%) of all cases of UGI bleeding, followed by variceal bleeding (10% or more) and Mallory-Weiss tears (5-10%). Vascular malformations are a less common but important cause and include angiomas or the rarer Dieulafoy's lesion (a superficial artery that erodes through the gut mucosa).
In some patients, bleeding is secondary to a coagulopathy. The most important current causes of this are liver disease and inadequately controlled warfarin therapy.
2. The cause of the haemorrhage may be obvious from the history, such as a long history of indigestion or, more significantly, previous haemorrhage from an ulcer. A history of aspirin or non-steroidal anti-inflammatory drug ingestion suggests acute ulceration. Approximately 85% of patients stop bleeding spontaneously within 48 hours. Haematemesis is the vomiting of blood. Melaena is the passage of black tarry stools; the black colour is due to altered blood – 50 mL or more is required to produce this. Melaena can occur with bleeding from any lesion from areas proximal to and including the caecum. Following a massive bleed from the upper gastrointestinal tract, unaltered blood (owing to rapid transit) can appear per rectum, but this is rare. The color of the blood appearing per rectum is dependent not only on the site of bleeding but also on the time of transit in the gut. With shock, remember that the peripheral arterial constriction that occurs may keep the blood pressure falsely high. Anaemia does not develop immediately as haemodilution has not taken place. Older patients have a higher risk of rebleeding. The severity of the initial hemorrhage has a direct correlation with the risk of rebleeding.
3. Profuse gastrointestinal bleeding may be life-threatening and constitutes a medical emergency that requires prompt treatment in a hospital environment. In the past, most situations like this required emergency surgery and removal of part of the stomach. Now with our newer technology, many bleeding ulcers can be treated endoscopically which stops the bleeding. In many cases, surgery can now be avoided.
4. Flexible endoscopy is the investigation of choice. Endoscopy should be performed as soon as practically possible. Endoscopy is able to detect and localize the site of the bleeding in 95% of cases and is clearly superior to contrast radiography (with an accuracy of only 75 to 80%). Endoscopy should be repeated to assess the bleeding site and to treat, if possible. The timing of subsequent endoscopy is dependent on two factors: the severity of the hemorrhage and the risk status of the patient. Most bleeding from upper gastrointestinal lesions can be effectively controlled endoscopically. Initial hemostasis can be achieved in 90% or more of cases; rebleeding, which may recur in up to 20% of cases, will respond about half of the time to a second endoscopic procedure. Endoscopic means of treating stress ulceration may be ineffective and operation may be required.
Endoscopic treatment methods:
injections with various substances (adrenalin or epinephrine, saline, absolute alcohol, sclerosants, thrombogenesis substances);
vessel’s thermal coagulation (heater probe, laser therapy, monopolar and multipolar electrocoagulation, argon plasma coagulation);
microwave probes;
mechanical methods (band ligation, endoscopic sewing machine, endoloops, and hemoclips);
fibrin glue sealing;
endoscopic cryotherapy;
combination therapy (combination of injection with dilute epinephrine and electrocoagulation, the heater probe plus thrombin injection etc.).
The success of endoscopic treatment depends on the size of the bleeding vessel. Large vessels (>1-2 mm) are difficult to treat with endoscopic methods. The relationship between effect and success of haemostasis and the individual endoscopic skill and technique of the endoscopist was confirmed.
Classification of bleeding activity (Forrest classification):
Bleeding activity |
Risk of further bleeding |
Type I or F1: Active bleeding Type Ia or F1a: active arterial bleeding (sputing hemorrhage) Type Ib or F1b: oozing bleeding |
High |
Type II or F2: Bleeding with recent signs Type IIa or F2a: non-bleeding visible vessel (visible vessel-pigmented protuberance) Type IIb or F2b: signs of recent bleeding (adherent clot, blood or coagula in upper GI tract) Type IIc or F2c: black ulcer base |
High or low |
High |
|
Low |
|
Type III or F3: no signs of recent bleeding (haematemesis or melaena in the past 48 h), clean ulcer base |
Low |
Wider use of endoscopic hemostasis in upper gastrointestinal bleeding has reduced significantly the need for operation. Failure to control bleeding endoscopically should not delay surgery when necessary, and a close cooperation between endoscopists and surgeons is essential.
5. All cases with a gastrointestinal bleed should be sent to hospital. Whatever the cause, the principles of management are identical. The following are factors that will affect the management:
аge;
the amount of blood lost, which may give some guide to the severity;
continuing visible blood loss;
presence of the classical clinical features of shock (pallor, cold nose, tachycardia and low blood pressure).
Urgent resuscitation is required in patients with large bleeds and the clinical signs of shock. The major principle is to rapidly restore the blood volume to normal. Prompt correction of hypovolaemia is necessary in patients with cirrhosis as their baroreceptor reflexes are diminished. For any significant gastrointestinal bleed, intravenous access should be established and, for those with severe bleeding, central venous pressure monitoring should be set up and bladder catheterisation performed. Blood should be cross-matched and the patient transfused as clinically indicated. The pulse rate and venous pressure are the best guides to transfusion rates.
Drug therapy: H2-receptor antagonists or proton-pump inhibitors, octreotide, antacids.
The coagulopathy may be corrected, if possible, with fresh-frozen plasma.
Surgery is necessary only if bleeding is persistent and/or uncontrollable. The advent of endoscopy has greatly helped in the management of upper gastrointestinal bleeding as a surgeon can usually be confident about the site of bleeding prior to operation.
Angiographic embolization of the gastroduodenal or the left gastric arteries may be effective, but there are dangers involved. A foreign body may slip from the gastroduodenal into the hepatic artery and lead to hepatic necrosis; one in the left gastric artery may lead to necrosis of the upper portion of the stomach.
6. Chronic peptic ulceration still accounts for approximately half of all cases of upper gastrointestinal haemorrhage. Patients who continue to bleed (typically patients with large ulcers in the posterior wall of the duodenal bulb) are usually managed angiographically (with embolization of the bleeding vessel) or surgically.
Acute ulceration.
Corticosteroids, aspirin and other non-steroidal anti-inflammatory drugs can undoubtedly produce acute gastric and duodenal ulcerations, particularly in the elderly. These agents are also responsible for gastrointestinal haemorrhage.
H. pylori have influence on gastrointestinal haemorrhage. Eradication of H. pylori is started as soon as. A proton-pump inhibitor is continued for four weeks to ensure healing.
Stress ulceration. This commonly occurs in patient, with major injury or illness, who have undergone, major surgery or who have major comorbidity. Many such patients are found in intensive care units. Prophylaxis of stress ulceration: acid inhibition and the nasogastric or oral administration of sucralfate.
The stigmata of a recent bleed (the patient is more likely to rebleed):
purting artery;
active oozing;
fresh or organized blood clot;
black spots.
At endoscopy. For ulcer bleeding the standard procedure consists of diagnostic emergency endoscopy and endoscopic treatment based on the bleeding activity. All bleeding ulcers should be either injected with adrenalin and a sclerosant or the vessel coagulated either with a heater probe or with laser therapy. These methods reduce the incidence of rebleeding, although they do not significantly improve mortality. Endoscopic application of metal clips to a bleeding vessel is a new alternative to injection therapy and thermal coagulation.
Surgical treatment: criteria for surgery are well worked out. The aim of the operation is to stop the bleeding. A patient who continues to bleed requires surgical treatment. The same applies to a significant rebleed. Patients with a visible vessel in the chronic ulcer base, a spurting vessel or a chronic ulcer with a clot in the base are statistically likely to require surgical treatment to stop the bleeding. Elderly and unfit patients are more likely to die as a result of bleeding than younger patients. Ironically, they should have early surgery. If the bleeding gastric ulcer is not excised then a biopsy of the edge needs to be taken to exclude malignant transformation. Various scoring systems have been devised, which predict the probability of rebleeding and mortality with some degree of accuracy. Early elective surgery is an effective procedure in bleeding peptic ulcer patients at high risk for rebleeding. Massive haemorrhage from a gastric ulcer is more likely to be fatal than that from a duodenal ulcer.
Common operations for bleeding duodenal ulcers: duodenoplasty with or without vagotomy, gastric resection according to Billroth I or II, truncal vagotomy with pyloroplasty. Gastric resection is more difficult, but gives slightly better control of bleeding because it removes areas of gastritis and duodenitis that are potential sources of postoperative bleeding.
Common operations for bleeding gastric ulcers. Gastric resection according to Billroth I is preferred. A truncal vagotomy should be added if the ulcer is in the prepyloric area or if the patient has a history of a duodenal ulcer. In some patients who are very poor operative risks, a local excision of the ulcer may be performed. However, the chances of recurrence within a year approach nearly 50 per cent.
7. Mallory-Weiss syndrome (synonyms: gastroesophageal laceration syndrome, gastroesophageal laceration-hemorrhage syndrome, retching erosion syndrome, Mallory-Weiss tear) is characterized by a mucosal gastric tear in the circular lining of the esophagus or stomach (at or near the point where the esophagus and stomach join) and produced by a sudden increase in intraabdominal pressure, prevailes in males, usually onset after 30 years of age. Mallory-Weiss syndrome is most often caused by repeated vomiting, an uneven distribution of pressure along the esohageal wall, hiatal hernia, or childbirth. Although Mallory-Weiss syndrome is not directly caused by alcohol, they are strongly related. Consuming large amounts of alcohol induces vomiting which brings stomach acids and fluids up into the esophagus. The acids wear away at the esophageal lining and weaken it. Vomiting also dramatically increases the pressure on the esophagus walls. Large amounts of food moving up the esophagus very quickly increases the chances of ripping or tearing the esophageal lining.
Presenting symptoms include
hematemesis (in all patients),
melena (10%),
light-headedness, dizziness, syncope (in patients with severe vomiting)
epigastric pain, symptoms of heartburn (these symptoms often are related to the underlying cause and not specifically to the Mallory-Weiss tear).
At endoscopy: a blood clot from a gastroesophageal tear. Endoscopy also shows that in 35% of patients there is another potential cause for gastrointestinal bleeding, such as peptic ulcer, erosive gastritis , or esophageal varices.
Signs are pallor; tachyardia; in some patients shock. The haemorrhage may be large but most patients stop spontaneously. Mallory-Weiss tears almost never rebleed; thus, follow-up usually is not indicated. When bleeding does not stop, patients are treated with an injection of epinephrine (adrenaline) and/or the bleeding artery is cauterized with heat. If these treatments fail, surgery is performed to stop the bleeding. Rarely, surgery with over-sewing of the tear will be required. Patients at highest risk for a recurrence of bleeding are those with portal hypertension.
8. Erosive gastritis has a variety of causes, especially NSAIDs. Fortunately, most bleeding settles spontaneously, but when it does not it can be a major problem to treat. In general terms, although there is a diffuse erosive gastritis, there is one (or more) specific lesion that has a significant-sized vessel within it. This should be dealt with appropriately, preferably endoscopically, but sometimes surgery is necessary.
9. Gastro-oesophageal variceal haemorrhage. With liver disease the bleeding is often severe and recurrent if it is from varices. Splenomegaly suggests portal hypertension but its absence does not rule out oesophageal varices. Liver failure can develop. Portal hypertensive (or congestive) gastropathy is the term used for chronic gastric congestion, punctate erythema and gastric erosions and is a source of bleeding. Approximately 70% of patients with cirrhosis will develop gastro-oesophageal varices, but only one-third of these will bleed from them. Bleeding is likely to occur with large varices, high pressure (>12 mmHg) and in severe liver disease.
The management can be divided into the active bleeding episode, the prevention of rebleeding, and prophylactic measures to prevent the first haemorrhage.
At endoscopy. Endoscopy should be performed to confirm the diagnosis of varices and to exclude bleeding from other sites (e.g. gastric ulceration), which are sometimes the source of haemorrhage in these patients. Hemostasis is achieved using either band ligation, sclerotherapy, or a combination of both. These methods reduce the incidence of rebleeding, although they do not significantly improve mortality. Gastric varices require surgical control since they are generally endoscopically inaccessible to treatment. Even if initial endoscopic hemostasis is successful, long-term prevention of rebleeding requires a program of ongoing endoscopic sessions until variceal obliteration is complete. Ligation is the preferred approach in this setting because it is associated with fewer side effects. Endoscopic variceal ligation technique:
the endoscope, with attached ligating device, is brought into contact with a varix just above the gastroesophageal junction;
suction is applied, drawing the varix-containing mucosa into the dead space created at the end of the endoscope by the ligating device;
the trip-wire is pulled, releasing the band around the aspirated tissue.
Vasoconstrictor therapy. The aim of vasoconstrictor agents is to restrict portal inflow by splanchnic arterial constriction.
Octreotide. This drug (a somatostatin analogue — 50 Jig bolus followed by an infusion of 50 jig per hour for 48 hours) will produce splanchnic vasoconstriction without significant systemic vascular effect or complications. Octreotide is safe, as effective as balloon tamponade, and is often used while awaiting sclerotherapy.
Vasopressin. An infusion of 25 units per hour should be administered by a central venous catheter (if possible) to avoid the risks of local leakage and necrosis. Vasopressin should not be given to patients with ischaemic heart disease. The addition of nitrates either by the intravenous, sublingual or patch route has been shown to enhance the efficacy of vasopressin and to reduce its complications. The patient will complain of abdominal colic, will defaecate and have facial pallor owing to the generalized vasoconstriction.
Terlipressin (2 mg bolus 6-hourly) is longer acting than vasopressin and is an alternative.
Balloon tamponade. This procedure is used mainly to control bleeding if sclerotherapy has failed or is unavailable, or if vasoconstrictor therapy has failed or is contraindicated. The tube should be left in place for up to 12 hours and removed in the endoscopy room prior to sclerotherapy. The usual tube is a Sengstaken-Blakemore. The tube is passed into the stomach and the gastric balloon is inflated with air and pulled back. It should be positioned in close apposition to the gastro-oesophageal junction to prevent the cephalad variceal blood flow to the bleeding point. The oesophageal balloon should be inflated only if bleeding is not controlled by the gastric balloon alone. This technique is successful in up to 90% of patients and is very useful in the first few hours of haemorrhaging. However, it has serious complications such as aspiration pneumonia, oesophageal rupture and mucosal ulceration, which lead to a 5% mortality. The procedure is very unpleasant for the patient.
Fifty per cent of patients with stopped variceal haemorrhage rebleed within 10 days. The source of the rebleed should be established by endoscopy. It is sometimes due to an ulcer produced by previous sclerotherapy and this is difficult to manage. Management of an acute rebleed starts with repeat sclerotherapy and octreotide infusion for 3-5 days. Following an episode of variceal bleeding, the risk of recurrence is 60-80% over a two-year period with an approximate mortality of 20% per episode. These facts justify the use of measures to prevent rebleeding:
β-blockers (e.g. propranolol) reduces the chances of variceal haemorrhage, may increase survival and is cost-effective (propranolol also is the best treatment for portal hypertensive gastropathy);
liver transplantation should always be considered.
Despite all the therapeutic techniques available, the prognosis depends on the severity of the underlying liver disease, with an overall mortality from variceal haemorrhage of 30% - reaching 50% in Child's grade C.
Transjugular intrahepatic portocaval shunt (TIPS). In this technique, a guidewire is passed from the jugular vein into the liver and an expandable metal shunt is forced over it into the liver substance to form a channel between the systemic and portal venous systems. It reduces the hepatic sinusoidal and portal vein pressure by creating a side-to-side shunt, but without the risks of general anaesthesia and major surgery. TIPS is used in cases where the bleeding cannot be stopped after two sessions of sclerotherapy 24 hours apart. It is useful in the short term, but recurrent portal hypertension owing to stent stenosis can occur within two years.
Emergency surgery. This is used when other measures fail or if TIPS is not available and, particularly, if the bleeding is from gastric fundal varices. Oesophageal transection and ligation of the feeding vessels to the bleeding varices is the most common surgical technique.
Oesophageal transection. The procedure is performed via a left subcostal or upper midline incision. The oesophagogastric junction is identified. The oesophagus is mobilized with its surrounding para-oesophageal tissues, from the oesophagocardiac junction to the upper margins of the diaphragmatic crus, a length of approximately 5 cm. A strong linen thread is passed around the mobilized distal oesophagus and a short anterior gastrotomy is made. After assessing the lumen of the oesophagus with passage of calibrated obturators, the appropriate size of staple gun (the Russian SPTU, Autosuture or Ethicon stapling guns) is passed into the oesophagus. The head is then separated from the anvil, and the linen thread tied tightly on to the shaft of the gun just above the oesophagogastric junction. After removal of any sling or tape around the oesophagus, the anvil and staple cartridge are approximated and the gun fired. In one manoeuvre, the oesophagus is transected with removal of a 1-cm ring of oesophagus, and the ends reanastomosed with a secure double layer of steel clips. The gun is opened and the doughnut inspected, a complete ring indicating a technically satisfactory transection. A nasogastric tube is carefully guided through the anastomosis and its tip placed in the body of the stomach before closure of the gastrotomy. The abdomen is closed without drainage, the patient allowed to take small sips of water, and the nasogastric tube usually removed after 2 days before allowing return to normal oral intake. This technically straightforward procedure can be performed within 1 to 2 h and usually with little blood loss. Varying degrees of devascularization can be added to the procedure. The most frequently employed operations are the Hassab devascularization and Sugiura devascularization with oesophageal transection.
The Hassab procedure consists of devascularization of the upper half of the stomach and oesophagus. The first step is usually splenic artery ligation followed by careful mobilization of the spleen. This mobilization as in all dissections in portal hypertension, requires patient ligation and coagulation of multiple collaterals within the peritoneal reflections, and after individual ligation and division of the short gastric vessels, the spleen is removed. The whole proximal stomach is then devascularized from the terminal two branches of the left gastric artery at the incisura angularis upwards by ligation and division of the lesser and greater omentum, and of the posterior gastric adhesions. After division of the oesophagogastric reflection of peritoneum and mobilization of the vagi, the distal 7 to 8 cm of oesophagus is mobilized and all feeding vessels are ligated and divided. Exposure in this part of the procedure is much facilitated by the use of costal margin retractors. The distal 3 cm of oesophagus and proximal 5 cm of stomach may then be opened longitudinally thus displaying the varices and allowing obliteration of each variceal column by undersewing from as high as possible within the oesophagus with an absorbable suture. After positioning of a nasogastric tube the oesophagogastrotomy is carefully closed by suturing or stapling.
The Sugiura operation is a much more radical development of the above method, classically performed in two staged procedures. At the first operation, via a left thoracotomy, the distal intrathoracic oesophagus is devascularized and an oesophageal transection performed. Six weeks later, via an upper abdominal midline incision, the intra-abdominal oesophagus and proximal stomach are devascularized by lesser and greater curve division and splenectomy. Vagotomy and pyloroplasty are then performed. This massive procedure has been modified into a one-stage operation using a transabdominal approach facilitated by the use of costal margin and sternal retractors. After division of the crura of the diaphragm, 10 cm of oesophagus can be devascularized, a staple transection performed via a gastrotomy and the rest of the abdominal part of the operation completed.
10. Dieulafoy's disease is a gastric arterio-venous malformation that has a characteristic histological appearance. The lesion itself is covered by normal mucosa and, when not bleeding, it may be invisible. If it can be seen while bleeding, all that may be visible is profuse bleeding coming from an area of apparently normal mucosa. If this occurs, the cause is instantly recognisable. If the lesion can be identified endoscopically there are various means of dealing with it, including injection of sclerosant. If it is identified at operation then only a local excision is necessary. Occasionally, a lesion is only recognised after gastrectomy and sometimes not even then. The pathologist, as well as the endoscopist, may have difficulty in finding it.
11. Gastric carcinoma. Most patients do not have a large bleeding.
12. Most re-bleeds (approximately 25% of all cases) occur within 48 hours. Below the age of 60 years mortality from gastrointestinal bleeding is small, but above the age of 80 the mortality is greater than 20%. Patients with recurrent haemorrhage have an increased mortality.
13. Gastrointestinal bleeding in the elderly is associated with greater morbidity and mortality than in the young. Sources of bleeding include those seen in younger patients as well as some that are unique to the elderly. As in younger people, the clinical presentation may vary from anemia without specific gastrointestinal symptoms to massive, life-threatening bleeding. Because of alterations in pain perception with age and atypical clinical presentations, certain gastrointestinal disorders can present differently in the elderly as compared with the young. As in younger patients, elderly patients with acute upper gastrointestinal bleeding usually present with hematemesis (50%), as opposed to a combination of hematemesis and melena (20%) or melena alone (30%). Unexplained syncope should raise the suspicion of gastrointestinal bleeding in the elderly. A large volume of blood may be lost into the gastrointestinal tract without immediate hematemesis or passage of fresh (or altered) blood per rectum. In patients older than age 50, hypovolemia from hemorrhage may precipitate ischemic events, including myocardial infarction, which may be silent. Any elderly patient who remains hypotensive and tachycardic despite apparently adequate volume replacement should be assessed for possible myocardial infarction.
Endoscopic treatment. There are no data to suggest that visible vessels in the elderly are more resistant to endoscopic therapy because of atherosclerosis of the underlying artery. The gastric wall may be dangerously thin in the elderly, however, especially in patients with gastric atrophy, particularly in the fundus and proximal body. [138] In such cases, injection of alcohol or other sclerosants should be avoided, especially in the fundus, to avoid delayed perforation.
Surgery should not be delayed because of old age. Early surgery has been recommended for elderly patients with major ulcer bleeding and endoscopic findings that imply a high risk of rebleeding, particularly active spurting, because emergent surgery has a higher morbidity and mortality rate than elective early surgery. The recommendation for early surgery in elderly patients is, however, controversial. Recent studies have favored repeating endoscopy for rebleeding before committing an elderly patient to surgery.
Peptic ulcer disease associated with ingestion of NSAIDs or gastric colonization by H. pylori is a more serious disorder in the elderly than in younger persons. Not only does the incidence of duodenal and gastric ulcer increase with age in both sexes, but also the incidence of complications, such as perforation and hemorrhage, and the mortality rate from peptic ulcer disease rise progressively with age.
Several features of ulcer disease are unique in the elderly. The so-called geriatric ulcer is often several centimeters in diameter; is located high in the cardia; and may cause dysphagia, mimicking an esophageal neoplasm, or may cause chest pain, mimicking angina. Giant duodenal ulcers (>2 cm in diameter or >60% of the duodenal bulb) typically occur in men older than age 70 and may be mistaken for the duodenal bulb itself on an upper gastrointestinal series. In the past, these ulcers were commonly fatal. Now most such ulcers heal with acid-suppressive therapy and eradication of H. pylori. Giant gastric ulcers greater than 3 cm in diameter usually are seen in persons older than age 65 and are commoner in men than women. Although typically benign, hemorrhage is frequent and often severe, with high morbidity and mortality rates.
Other important causes of upper gastrointestinal bleeding in the elderly include aortoenteric fistulas, Dieulafoy's lesions, gastric antral vascular ectasia, and gastroduodenal arteriovenous malformations. Aortoenteric fistulas occur most often in elderly men, especially those who had aortic surgery in the past. A high index of suspicion for the diagnosis is required in any patient with an aortic graft and a herald bleed, which presages exsanguinating hemorrhage. Diagnosis is by upper endoscopy or computed tomography initially. Therapy of gastric antral vascular ectasia includes endoscopic ablation with neodymium-yttrium-aluminum-garnet (Nd:YAG) or argon laser and surgery. Arteriovenous malformations may also be treated by endoscopic hemostasis.
Theme: ACUTE LOWER GASTROINTESTINAL BLEEDING
KEY QUESTIONS FOR HOMEWORK:
Causes of hemorrhage of the intestinal tract.
Clinical signs and diagnostics.
Lower gastrointestinal bleeding in the elderly.
Frequent causes of hemorrhage of the lower digestive tract:
Anorectal diseases
Polyps of the colon
Diverticular disease of the colon (multiple diverticulae of the colon)
Colon and rectal cancer
Intestinal amebiasis
Ulcerative colitis
Regional enteritis
Arterio-venous malformation.
Massive bleeding from the lower gastrointestinal tract is rare. On the other hand, small bleeds from haemorrhoids occur very commonly. Massive bleeding is usually due to diverticular disease or ischaemic colitis and may require urgent resuscitation. The most common cause of acute lower gastrointestinal bleeding is angiodysplasia, followed by diverticulosis, neoplasms, and colitis.
Surgery is rarely required as bleeding usually stops spontaneously.
2. In contrast to upper gastrointestinal bleeding, there is no single best test for acute lower gastrointestinal bleeding. At the outset, a careful digital rectal examination and sigmoidoscopy should be done to exclude anorectal pathology and to confirm the patient's description of the symptoms. In young patients (<40 years) with minor bleeding, features that are highly suggestive of anorectal origin (e.g., blood on the surface of the stool or on the wipe) may warrant only a flexible sigmoidoscopy. Conversely, patients presenting with hemodynamic compromise may need an upper endoscopy first to exclude a lesion in the upper gastrointestinal tract (typically post-pyloric) that is bleeding so briskly that it presents as hematochezia (which may be seen in 10% or more of acute upper gastrointestinal bleeding). Colonoscopy has traditionally been recommended after bleeding has slowed or stopped and the patient has been given an adequate bowel purge. However, a disadvantage of delaying endoscopy is that when a pathologic lesion such as an arteriovenous malformation or diverticulum is found, it may be impossible to implicate it confidently as the site of bleeding (complementary information by radiography or scintigraphy becomes particularly important in this situation). Some experts therefore recommend an urgent diagnostic endoscopy with little or no preparation for acute lower gastrointestinal hemorrhages and have reported success rates of 50%.
Unspecific ulcerative colitis. Multiple serpentine ulcers, exudate, and edema are seen. Note the presence of pseudo-polyps and the diminished vascular markings.
A diagnosis is made using the following investigations as appropriate:
• rectal examination (e.g. carcinoma)
• proctoscopy (e.g. haemorrhoids)
• sigmoidoscopy (e.g. inflammatory bowel disease)
• colonoscopy – for any mucosal lesion and removal of polyps
• angiography – vascular abnormality (e.g. angiodysplasia).
3. Lower gastrointestinal bleeding in the elderly. Eliciting a medical history and identifying pertinent risk factors help in determining the cause of lower intestinal bleeding in the elderly. Aspirin or NSAID use is strongly associated with lower gastrointestinal bleeding, particularly diverticular bleeding. Bleeding associated with antecedent hypovolemia should raise the possibility of ischemic colitis, whereas prior radiation therapy for prostate or pelvic cancer suggests radiation proctitis, which can present months or years after radiation. A history of severe constipation should raise the possibility of a stercoral ulcer, and a recent colonoscopic polypectomy suggests postpolypectomy bleeding.
Lower intestinal bleeding stops spontaneously in most cases (80% to 85%).
Endoscopic therapy is applied to lower gastrointestinal bleeding in 12% to 27% of cases. Modes of endoscopic therapy for acute lower intestinal bleeding, in particular, for angiodysplasia and diverticular disease, include thermal contact probes, laser, monopolar electrocautery (hot biopsy forceps), injection sclerotherapy, and band ligation.
Surgery. Age, probably by association with increased comorbidity, is an important risk factor for postoperative mortality. Accurate preoperative localization of the bleeding site is essential for successful segmental colonic resection.
Colonic diverticula account for 42% to 55% of cases of lower intestinal hemorrhage in the elderly. Bleeding seldom complicates diverticulitis, and when it does, the bleeding is typically occult or minor. More serious diverticular bleeding tends to be from the right colon. Because bleeding from diverticula ceases spontaneously in 80% of cases, emergent surgery, with its increased morbidity and mortality, is usually avoided. A conservative approach to therapy is strongly recommended. Once bleeding has stopped and the patient has been fully evaluated and prepared, surgery, when indicated, is much safer, especially in older patients. Because most diverticula do not bleed repeatedly, patients with resolved lower gastrointestinal bleeding originating from a diverticulum can be discharged without surgery.
Angiodysplasia are acquired lesions of aging that result from dilation and tortuosity of the submucosal veins caused, in part, by increased colonic intraluminal pressure. Angiodysplasia occur with equal frequency in men and women. More than half are located in the cecum and proximal ascending colon. They are usually multiple, measure less than 5 mm in diameter, and bleed predominantly from the right colon. Angiodysplasia are responsible for 3% to 12% of cases of acute lower intestinal bleeding. In more than 90% of cases, bleeding stops spontaneously. Angiodysplasia may be identified by colonoscopy, as a red, flat lesion, about 2 to 10 mm, sometimes with a feeding vessel or a clearly visible pale mucosal halo, or by angiography, as an ectatic slowly emptying vein, vascular tuft, or early filling vein.
Colonic ischemia. Ischemic colitis, the commonest ischemic disorder of the gastrointestinal tract, causes about 3% to 9% of cases of acute lower intestinal bleeding. Most often, vascular occlusion or a precipitating event cannot be identified. The greater frequency of ischemic colitis in the elderly as compared with the young suggests a relationship to degenerative changes in the vascular tree, but angiography rarely demonstrates significant abnormalities and plays little role in the evaluation of suspected colonic ischemia. Vascular atheromatous changes are almost universal in the elderly and are of uncertain pathogenic significance in ischemic colitis.
Colonic ischemia typically presents with the sudden onset of mild lower abdominal crampy pain accompanied or followed within 24 hours by lower intestinal bleeding or bloody diarrhea. Patients suspected of having colonic ischemia should undergo gentle colonoscopy or barium enema as the initial diagnostic test. Ulcerative lesions at colonoscopy are suggestive of ischemia. Biopsy shows necrosis, in contrast to the findings seen in ulcerative colitis.
Inflammatory bowel disease may present for the first time in the elderly, with bleeding and bloody diarrhea. Ulcerative colitis is more often restricted to the rectum and sigmoid colon in older patients than in younger patients. The presence of blood in the stool, particularly small amounts passed persistently; perianal disease; extracolonic manifestations; and proctitis on sigmoidoscopy are suggestive of Crohn's disease. There are several important therapeutic considerations in treating older patients with inflammatory bowel disease. Opiate-containing drugs may be relatively ineffective in the elderly because the elderly have higher endogenous serum opioid concentrations than younger people. In addition, oversedation leading to unsteadiness and consequent falls should be avoided in the elderly. Loperamide has fewer central effects and is often better tolerated than diphenoxylate with atropine. Anticholinergic agents should be avoided because of potential cardiac complications, mental changes, and gastrointestinal hypomotility. Sulfasalazine, mesalamine, azathioprine, and 6-mercaptopurine are all tolerated well in elderly patients. Metronidazole can interfere with the oxidation of warfarin and might induce excessive anticoagulation; monitoring the prothrombin time is required. Corticosteroids have a higher risk of complications in the elderly than in the young, including osteopenia, hyperglycemia, cataract formation, and behavioral changes. Although corticosteroids are the agents of choice for the treatment of severe attacks in the elderly as in the young, these drugs should be reduced in dose or discontinued as soon as the patient's clinical condition improves.
Less common sources of lower gastrointestinal bleeding in the elderly:
certain infections are common in the elderly, including Salmonella and Escherichia coli;
radiation proctitis (for 1% to 5% of cases of acute lower intestinal bleeding);
hemorrhoids;
stercoral ulcers (These ulcers develop under an adherent mass of hard stool and occur most frequently in the rectosigmoid and sometimes in the transverse colon, where fecalomas also occur. Stercoral ulcers may be the source of massive hemorrhage);
benign and malignant neoplasms;
intussusception, which is typically accompanied by crampy abdominal pain and bloody stools with the consistency of currant jelly;
trauma, from an endoscopic procedure or from an enema catheter;
solitary rectal ulcer syndrome;
portal colopathy;
colonic varices;
endometriosis;
Dieulafoy lesion of the colon;
bleeding from the appendiceal orifice.
Theme: ACUTE GASTROINTESTINAL HEMORRHAGE FROM AN OBSCURE SOURCE
KEY QUESTIONS FOR HOMEWORK:
Definition.
Small intestinal hemorrhage (etiology, diagnostic and treatment).
Rare causes of gastrointestinal hemorrhage from an obscure source.
Obscure bleeding. Recurrent or persistent iron-deficiency anemia, positive fecal occult blood test or visible bleeding with no bleeding source found at original endoscopy.
Obscure-occult bleeding. Subcategory of obscure bleeding characterized by recurrent or persistent iron-deficiency anemia and/or positive fecal occult blood test with no source found at original endoscopy; no visible blood in feces.
Obscure-overt bleeding. Subcategory of obscure bleeding characterized by recurrent or persistent overt / visible bleeding with no source found at original endoscopy; bleeding manifest as visible blood in emesis or feces.
Small intestinal hemorrhage. The small bowel is a rare source of acute hemorrhage. Only 2 to 5% of patients with acute gastrointestinal hemorrhage are ultimately determined to have bled from a small intestinal source. This low frequency is fortunate because the small bowel is a difficult organ to visualize and precise detection of the bleeding lesion is characteristically delayed. Small bowel hemorrhage is frequently episodic, characterized by recurrent brisk hemorrhage, which ceases spontaneously only to recur weeks or months later. The typical patient referred with acute gastrointestinal hemorrhage of obscure origin had intermittent episodes of hemorrhage during a 26-month period, had undergone 1 to 20 diagnostic tests, and had received an average of 20 units of packed red blood cells before diagnosis (A.Szold et al., 1992).
The clinical presentation of patients with acute small bowel hemorrhage is similar to that of acute lower gastrointestinal hemorrhage from a colonic source. Initial priorities in management are resuscitation followed by prompt diagnostic testing: emergency upper endoscopy in hemodynamically unstable patients with hematochezia and nasogastric aspiration in patients with melena, followed promptly by selective visceral angiography or emergency colonoscopy. In the rare patient with significant, ongoing small bowel bleeding at the time of these initial evaluations, a positive source may be identified. Colonoscopy may reveal hemorrhage coming through the ileocecal valve or pooling in the terminal ileum, whereas selective mesenteric angiography may reveal the bleeding source from any of many possible small bowel sources. Primary surgical exploration without investigation is to be condemned because the surgeon is rarely able to identify the actual source of small intestinal bleeding by visual inspection or palpation.
Etiology and Treatment.
Angiodysplasias are responsible for 50 to 75% of cases of hemorrhage from the small bowel in patients older than 50 years of age and for 30 to 40% of cases in younger patients. These acquired lesions are most common in the right colon but can be found throughout the gastrointestinal tract. Bleeding is characteristically episodic, making diagnosis difficult. The lesions can be identified with angiography or enteroscopy. An effort at endoscopic sclerotherapy or coagulation is indicated in patients diagnosed endoscopically before surgical exploration. In all others and in those in whom endoscopic measures fail, surgical segmental resection of the small bowel is indicated. Because angiodysplasias are acquired with age, up to 25% of patients develop new hemorrhage from other angiodysplasias in subsequent years; thus, long-term follow-up is required.
The telangiectasias of Osler-Weber-Rendu syndrome are distinct from angiodysplasias. Most patients with this hereditary disorder present with skin and oral lesions in youth and acquire gastrointestinal lesions with bleeding in the fourth decade of life, or later. These telangiectasias are diffuse, and surgical intervention is not appropriate. Some success with conjugated estrogen therapy to decrease the frequency and degree of hemorrhage has been reported.
Neoplasms are the second most common cause of small intestinal hemorrhage. Most are benign, including leiomyomas, schwannomas, and lipomas, although malignant tumors may also bleed, including leiomyosarcoma, adenocarcinoma, lymphoma, carcinoid tumors, and others. Central necrosis of the tumor is the cause of bleeding. Therapy is surgical resection.
Meckel's diverticulum is a remnant of the vitelline duct and is found within the final 100 cm of ileum in up to 2% of the population. It is the most common cause of hemorrhage from the small bowel in patients younger than 30 years of age. Bleeding may be brisk, and in young patients, prompt exploratory laparotomy after a negative upper endoscopy is appropriate for those with ongoing hemorrhage and hemodynamic instability. Meckel's scan is positive in about 60% of patients. Surgery to remove the diverticulum is required in a patient who has had hemorrhage.
Jejunal diverticula are acquired pseudodiverticula that develop within the leaves of the small bowel mesentery. These diverticular are present in 1 to 2% of patients, and it is estimated that hemorrhage develops in 5% of cases. Bleeding is identified with angiography, which can show pooling of the extravasated blood in the diverticulum, or at endoscopy. Surgical resection is the optimal therapy.
3. Rare causes of gastrointestinal hemorrhage from an obscure source.
Acute gastrointestinal hemorrhage from an obscure source has been reported to occur from a variety of conditions. These include erosions within hiatal hernias (Cameron’s erosions), radiation enteritis, small intestinal varices, Crohn's disease, tuberculosis, syphilis, typhoid, histoplasmosis, small bowel ulcerated lesions in patients with gastrin-secreting tumors, celiac sprue, Dieulafoy's lesions, and Osler-Weber-Rendu syndrome. Medical treatment is appropriate for most infectious causes and in patients with Zollinger-Ellison syndrome. Enterectomy is required in the other conditions.
Disorders of the pancreas can cause acute gastrointestinal hemorrhage as blood is delivered into the duodenum through the pancreatic duct. Such bleeding has been reported in the setting of acute pancreatitis, chronic pancreatitis with rupture of pancreatic micro-pseudoaneurysms after pancreatectomy, and pancreatic tumors. Bleeding is a rare complication of these disorders. Angiography may confirm the presence of a pseudoaneurysm and allow angiographic embolization for acute hemorrhage control. Pancreatic resection may be appropriate, depending on the clinical condition.
The liver may also be the source of presumed acute gastrointestinal hemorrhage. Bleeding into the hepatic duct presents as gastrointestinal hemorrhage as blood enters the duodenum from the common bile duct, a condition known as hemobilia. Hemobilia has been reported to occur secondary to hepatic trauma with intrahepatic hematoma, hepatic aneurysms or other vascular malformations, hepatic tumors, hepatic abscess, or after hepatic resection or percutaneous liver biopsy. This diagnosis is usually considered when endoscopic visualization during acute hemorrhage shows blood entering the duodenum at the ampulla of Vater depending on the clinical scenario. Selective visceral angiography is usually required to define the source and often allows definitive management by intra-arterial embolization.
Theme: CHRONIC GASTROINTESTINAL BLEEDING
KEY QUESTIONS FOR HOMEWORK:
Definition.
Clinical approach to the diagnostics.
Treatment
1. Chronic gastrointestinal bleeding – occult gastrointestinal bleeding. Initial presentation of iron-deficiency anemia and/or positive fecal occult blood test; no visible blood in feces.
2. Clinical approach to the diagnostics. Chronic blood loss can occur with any lesion of the gastrointestinal tract that produces acute bleeding. In addition, a Meckel's diverticulum and carcinoma of the caecum may present with an iron-deficiency anaemia. Vascular malformations may be found in 6% or more of patients. Careful history and examination may indicate the most likely site of the bleeding, but if no clue is available it is usual to investigate both the upper and lower gastrointestinal tract endoscopically at the same session ('top and tail'). Normal fecal blood loss is usually less than 2 to 3 mL/day. Most standard fecal occult blood tests will detect blood loss of 10 mL/day or more. For practical reasons an upper gastrointestinal endoscopy is performed first as this takes minutes only, followed by colonoscopy when any lesion can be removed or biopsied. A barium enema is performed only if colonoscopy is unavailable. Following negative investigations, an angiography may show the site of bleeding, particularly when acute bleeding is occurring. Occasionally intravenous technetium-labelled colloid may be used to demonstrate the bleeding site in a Meckel's diverticulum. Endoscopes to visualize the whole of the small bowel (enteroscopy - the use of a very long upper endoscope to intubate the small bowel) are available at specialist centres. If the patient continues to have symptomatic bleeding, enteroscopy may be helpful to detect small bowel angiomata.
3. Treatment. The cause of the bleeding should be treated, if found. Oral iron is given to treat anaemia
TESTS
1. Which of the following is better indicator of need for transfusion -
a) Urine output
b) Haematorcrit
c) Colour of skin
d) Clinical examination
2. Cryoprecipitate is a rich source of -
a) Thromboplastin
b) Factor VIII
c) Factor X
d) Factor VII
3. More than three units of blood transfusion is supplemented by intravenous injection of -
a) Furosemide
b) Calcium gluconate
c) Sodium bicarbonate
d) Multivitamin
4. Massive blood transfusion is defined as –
a) 350 ml in 5 min
b) 500 ml in 5 min
c) 1 Litre in 5 min
d) Whole blood volume
5. Hypovolaemic shock manifests when the percent of blood loss exceeds -
a) 10%
b) 15%
c) 25%
d) 30%
e) 40%
6. Following is the most important factor in the management of shock -
a) Blood pressure
b) Cardiac output
c) CVP to 8cm of water
d) Deficiency of effective circulation
7. Mallory-Weiss syndrome often occurs in -
a) Patient who abuse analgesics
b) Children
c) Pregnant females
d) Patients with hiatus hernia
8. A 26 year old women in the first trimester of pregnancy has been admitted with retching and repeated vomiting with large haematemesis. Her pulse rate is 126/minute and blood pressure is 80 mm Hg systolic. The most likely diagnosis is -
a) Mallory-Weiss syndrome
b) Bleeding from oesophageal varices
c) Peptic ulcer
d) Hiatus hernia
9. In Mallory-Weiss syndrome, where is the mucosal tear located -
a) Gastric cell anaplastic
b) Squamous cell CA
c) Gastro-oesophagal junction
d) Near gastric pylorus
10. The commonest cause of hematemesis and melena -
a) Oesophageal varies
b) Chronic peptic ulcer
c) Acute peptic ulcer
d) Carcinoma of stomach
11. The most useful method to diagnose cause of upper gastrointestinal haemorrhage is –
a) Barium study
b) Celiac angiography
c) Gastric biopsy
d) Endoscopy
12. All of the following are common causes of haematemisis except -
a) Chronic peptic ulcer
b) Pernicious anemia
c) Esophageal varices
d) Carcinoma of stomach
13. Uncommon cause of upper gastrointestinal bleeding -
a) Varices
b) Erosive gastritis
c) Peptic ulcer
d) Carcinoma stomach
14. Curlings ulcer is seen in -
a) Burns patients
b) Patients with head injuries
c) Zollinger Ellison syndrome
d) Analgesic drug abuse
15. Haemorrhage from a bleeding duodenal ulcer is due to the erosion of.......... artery -
a) Superior pancreatic duodenal
b) Gastroduodenal
c) Right gastric
d) Left gastric
16. Commonest stomach tumour which bleeds -
a) Adenocarcinoma
b) Squamous carcinoma
c) Leimysarcoma
d) Fibrosarcoma
17. Gastrin levels are raised in -
a) Zolinger Ellison syndrome
b) Pernicious anaemia
c) Gastric ulcer
d) All
18. The treatment of peptic ulcer involves -
a) Antacids
b) Ranitidine
c) Sucralfate
d) All
19. G-cells are present mostly in -
a) Fundus
b) Cardia
c) Pyloric antrum
d) Body
20. Gastric Acid secretion is inhibited by -
a) Gastrin
b) Alkaline pH
c) Vagal stimulation
d) Somatostatin
21. Anemia is greater in which of the following gastric resection -
a) Billroth II
b) Billroth I
c) Both of the above are equal
d) Neither of the above
22. Gastrin secretion by pyloric antrum is stimulated by -
a) Cholecystokinin
b) Alteration in pH of gastric contents
c) Distension of antrum
d) All of the above
e) None of the above
23. Presenting symptom of carcinoma stomach is -
a) Bleeding
b) Obstruction
c) Perforation
d) Weight loss
24. What complication commonly occurs in anterior duodenal ulcer -
a) Bleeding
b) Penetration
c) Perforation
d) Stricture formation
25. Bleeding in duodenal ulcer is from which artery -
a) Gastroduodenal
b) Splenic
c) Lesser gastric
d) Left gastric
26. The operation wherein the stump of the stomach is directly anastomosed to the stump of the duodenum is called -
a) Polya gastrectomy
b) Hoffmeister gastrectomy
c) Billroth 1 gastrectomy
d) Billroth II gastrectomy
27. Which of the following is not true of Curling's ulcer -
a) Seen in burned patients
b) Are solitary penetrating ulcer
c) Are shallow multiple erosions
d) Has also been described in children after head injury or craniotomy
28. Maximal reduction in gastric acidity is achieved by -
a) Trancal vagotomy and pyloroplasty
b) Truncal vagotomy and antrectomy
c) Partial gastrectomy
d) Highly selective vagotomy
29. Hemobilia is characterised by -
a) Jaundice
b) Biliary colic
c) Melena
d) Fever
30. Commonest cause of hemobilia is -
a) Gall stones
b) Trauma
c) Cholangitis
d) Hepatoma
31. Bleeding in a case of obstructive jaundice is treated with -
a) Fresh frozen plasma
b) Cryoprecipitate
c) Whole blood
d) Buffy coat extract
Answers
-
Test
Answer
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
a
b
a
d
e
c
a,c,d
a
c
b
d
b
d
a
b
c
a,b
d
c
d
a
c
d
c
a
c
b,d
b
a,b,c
b
a
References
Farrell J.J., Friedman L.S. Gastrointestinal bleeding in older people // Gastroenterology Clinics – 2000. – Vol.29. – No.1, Mar.
Forrest J., Finlayson N., Shearman D. Endoscopy in gastrointestinal bleeding // Lancet. – 1974. – II. – P. 394-397.
Imhof M., Ohmann C., Röher H.-D., Glutig H. Endoscopic versus operative treatment in high-risk ulcer bleeding patients – results of a randomised study // Langenbecks Arch Surg. – 2003. – Vol.387. – P. 327-336.
Oxford Textbook of Surgery. – http://med-lib.ru/english/index.shtml
Quadri A., Nimish Vakil Peptic ulcer bleeding: clips, heat, and outcome // Am J Gastroenterol. – Vol.97. - P. 200-201.
Schoenberg M.H. Surgical therapy for peptic ulcer and nonvariceal bleeding // Langenbecks Arch Surg. – 2001. – Vol.386. – No.2, Mar. – P. 98-103.
Short Practice of Surgery / Edited by R.C.G.Russell, Norman S. Williams, Christopher J.K. Bulstrode. – 24 th Edition. – Chapter 62, chapter 68. – P. 1026-61, 1153-85.
Zuckerman G.R., Prakash C., Askin M.P., Lewis B.S. AGA technical review on the evaluation and management of occult and obscure gastrointestinal bleeding // Gastroenterology. – 2000. – Vol.118. – No.1, Jan.
ГОУ ВПО «Смоленская государственная медицинская академия
Федерального агентства по здравоохранению и социальному развитию»
МЕТОДИЧЕСКИЕ УКАЗАНИЯ ДЛЯ СТУДЕНТОВ
ПО ДИСЦИПЛИНЕ хирургические болезни
ABDOMINAL INJURIES (trauma abdomen). COMBINATED THORACIC AND ABDOMINAL TRAUMA (thoracoabdominal trauma)
Составитель ассистент А.Л.Буянов
Методические указания утверждены на методическом совещании кафедры госпитальной хирургии (протокол № 2 от 6 октября 2008 г.)
Зав. кафедрой______________(проф. С.А.Касумьян)
2008 г.
Liver trauma
General
Liver injuries are fortunately uncommon because of the liver’s position under the diaphragm protected by the chest wall. However, when they do occur they are serious injuries associated with a significant morbidity and mortality, even with prompt and appropriate management. Liver trauma can be divided into blunt traumatic injuries which produce contusions, lacerations and avulsion injuries to the liver, and penetrating injuries, such as stab and gunshot wounds. The liver is typically one of many organs to be damaged, the chest or pericardium often being involved with penetrating injuries and the spleen or kidney with blunt trauma.
Diagnosis of liver injury
The liver is an extremely well-vascularised organ and blood loss is therefore the major early complication of liver injuries. Clinical suspicion of a possible liver injury is essential as a laparotomy by an inexperienced surgeon with inadequate preparation preoperatively is doomed to failure.
All lower chest and upper abdominal stab wounds should be suspect, especially if considerable blood volume replacement has been required.
Similarly, severe crushing injuries to the lower chest or upper abdomen often combine rib fractures, haemothorax and damage to the spleen and/or liver. Patients with a pene-trating wound will require a laparotomy and/or thoracotomy once active resuscitation is underway. Owing to the oppor-tunity for massive ongoing blood loss and the rapid devel-opment of a coagulopathy, the patient should be directly transferred to the operating suite whilst blood products are obtained and volume replacement is ongoing. Patients with blunt trauma who are haemodynamically stable but have objective clinical signs, such as upper abdominal tenderness and guarding, should have an oral and intravenous contrast enhanced CT scan of the chest and abdomen. This will demonstrate evidence of parenchymal damage to the liver or spleen as well as associated traumatic injuries to their feeding vessels. Free fluid can also be clearly established and a diagnostic aspirate performed. The chest scan will help to exclude injuries to the great vessels and demonstrate damage to the lung parenchyma. Additional investigations which may be of value include peritoneal lavage, which can confirm the presence of haemoperitoneum, and laparoscopy, which can demonstrate an associated diaphragmatic rupture.
Initial management of liver injuries
Penetrating
The initial management of a patient with an upper abdominal penetrating injury is the basis of resuscitation. The initial survey assesses the patients airway patency, breathing pattern and circulation. Peripheral venous access is gained with two large-bore cannulae and blood sent for cross match of 10 units of blood, full blood count, urea and electrolytes, liver function tests, clotting screen, glucose and amylase. Initial volume replacement should be with colloid or 0-negative blood if necessary. Arterial blood gases should be obtained and the patient intubated and ventilated if the gas exchange is inadequate. Intercostal chest drains should be inserted if associated pneumothorax or haemothorax is suspected. Once initial resuscitation has been commenced the patient should be transferred to the operating theatre with further resuscitation being performed on the operating table. The necessity for fresh frozen plasma and cryoprecipitate should be discussed with the blood transfusion service immediately the patient arrives, as these patients rapidly develop irreversible coagulopathies due to a lack of fibrinogen and clotting factors. Standard coagulation profiles are inadequate to evaluate this acute loss of clotting factors, and factors should be given empirically, aided by the results of thromboelastography (TEG), if available.
Blunt trauma
With severe blunt injuries the plan for resuscitation and management is as outlined above for penetrating injuries. For the patient whose vital signs are normal, imaging may be performed to evaluate further the nature of the injury. The basic surgical management differs between penetrating and blunt injuries thought to involve the liver. Penetrating injuries should be explored, whereas blunt injuries can be treated conservatively. The indication for discontinuing conservative treatment for blunt trauma would be evidence of ongoing blood loss despite correction of any underlying coagulopathy and the development of signs of generalised peritonitis.
The surgical approach to liver trauma
The surgical approach is partly dictated by the nature of the suspected injury. Good access is vital. A rooftop incision gives excellen isualisation of the liver and spleen and, if necessary, can be extended upwards for a median sternotomy. A stab incision in the liver can be sutured with a fine absorbable monofilament suture. If necessary, this may be facilitated by producing vascular inflow occlusion by placing an atraumatic clamp across the foramen of Winslow (the Pringle manoeuvre). Lacerations to the hepatic artery should be identified by placing an atraumatic bulldog clamp on the proximal vessel prior to repair with 5/0 or 6/0 Prolene suture. If unavoidable the hepatic artery may be ligated, although parenchymal necrosis and abscess formation will result in some individuals. Portal vein injuries should be repaired with 5/0 Prolene, again with exposure of the vessel being facilitated by the placement of an atraumatic vascular clamp. The blunt trauma of deceleration injuries often produces lacerations of the liver parenchyma adjacent to the anchoring ligaments of the liver. These may be amenable to suture with an absorbable monofilament suture. Again, inflow occlusion may facilitate this suturing and, if necessary, the sutures can be buttressed to prevent them cutting through the liver parenchyma. With more severe deceleration injuries a portion of the liver may be avulsed from anterior to posterior. These injuries are more complex as they are associated with a devitalised portion of the liver and often major injuries to the hepatic veins and IVC. The initial management of liver injuries is to pack the liver to produce haemostasis. This is effective for the majority of liver injuries if the liver is packed against the natural contour of the diaphragm by packing from below Large abdominal packs should be used to ease their removal, and the abdomen closed to facilitate compression of the parenchyma. Care should be taken to avoid over-zealous packing as this may produce pressure necrosis on the liver parenchyma. Crush injuries to the liver often result in large parenchymal haematomas and diffuse capsular lacerations. Suturing is usually ineffective, and packing is the most useful method of providing haemostasis. Necrotic tissue should be removed, but poorly perfused but viable liver left in situ. If packing is necessary the patient should have the packs removed after 48 hours, and commonly no further surgical intervention is required. Antibiotic cover is advisable and full reversal of any coagulopathy is essential.
If a major liver vascular injury was suspected at the time of the initial laparotomy then referral to a specialist centre should be considered. A common surgical approach under these circumstances would be to place the patient on veno-venous bypass using cannulae in the femoral vein via a long saphenous cutdown and being returned, via a roller pump, to the superior vena cava (SVC) via an internal jugular line.
Veno-venous bypass allows the IVC to be safely clamped to facilitate caval or hepatic vein repair. A rapid-infuser blood transfusion machine facilitates the delivery of a large volume of blood instantaneously. Once prepared, the patient is re laparotomised via the rooftop incision with a midline extension to the xiphisternum. The liver is mobilised by division of the supporting ligaments, and complete vascular isolation of the liver achieved by occluding the hilar inflow and the IVC above the renal veins and at the level of the diaphragm with atraumatic vascular clamps.
Venous return is provided by the veno-venous bypass. Warm ischaemia of the liver is tolerated for up to 45 minutes, allowing sufficient time in a blood-free field for repair of injuries to the IVC or hepatic veins.
Other complications of liver trauma
By far the most important complication of blunt or penetrating trauma to the liver is sudden massive blood loss. There are, however, other presentations and complications which require specific investigation and treatment. A subcapsular or intrahepatic haematoma requires no specific intervention and should be allowed to resolve spontaneously. Attempts to aspirate these lesions may result in the development of a liver abscess due to contamination. Abscesses may also form as a result of secondary infection of an area of extensive parenchymal ischaemia, especially after penetrating trauma. Treatment under these circumstances is with appropriate systemic antibiotics and aspiration under ultrasound guidance once the necrotic tissue has liquefied. Biliary fistulae are a rare but important complication of liver trauma and may be difficult to control. The main aspects to management are to drain any intraperitoneal bile collections externally by percutaneous drainage under ultrasound guidance. This is followed by endoscopic or percutaneous cholangiography to determine the site of the biliary fistula and decompress the biliary tree by nasobiliary drainage or endoprosthesis insertion. If this fails to control the fistula the affected portion of the liver may require to be resected. Late vascular complications include hepatic artery aneurysms and arteriovenous and arteriobiliary fistulae. These are best treated nonsurgically by a specialist hepatobiliary interventional radiologist. The feeding vessel can be embolised transarterialy. Evidence of liver failure may be seen with extensive liver trauma. If the blood supply and biliary drainage of the liver are intact this will usually reverse with conservative supportive treatment.
Long-term outcome of liver trauma
The capacity of the liver to recover from extensive trauma is remarkable, and parenchymal regeneration occurs rapidly. Late complications are rare but the development of biliary tract strictures many years after recovery from liver trauma has been reported. The treatment depends on the mode of presentation and the extent and site of stricturing. A segmen-tal or lobar stricture associated with atrophy of the corresponding area of liver parenchyma and compensatory hypertrophy of the other liver lobe may be treated expectantly. A dominant extra hepatic bile duct stricture associated with obstructive jaundice may be treated initially with endo-biliary balloon dilatation or stenting but will usually require surgical correction using a Roux-en-Y hepatodocho-jejunostomy.
The mesentery
A wound of the mesentery can follow a severe abdominal contusion and is a cause of haemoperitoneum.
Seat-belt syndrome If a car accident occurs when a seat belt is worn, sudden de-acceleration can result in a torn mesentery. This possibility should be borne in mind particularly as multiple injuries may distract attention from this injury. If there is any bruising of the abdominal wall, or even marks of clothing impressed into the skin, laparotomy may be indicated.
Diagnostic peritoneal lavage Diagnostic peritoneal lavage may be helpful in this situation (Chapter 4). Under local anaesthetic a subumbilical incision is made down to the peritoneum in a similar way to that used for ‘open’ laparoscopy (see above). A purse-string suture is placed in the peritoneum which is then incised. Free fluid, e.g. blood or intestinal contents, may be found, but if not a peritoneal dialysis catheter is inserted and the purse-string suture tied. A litre of normal saline is run into the peritoneum and then drained off by placing the bag and tubing below the patient’s abdomen. The presence of blood (>100 000 red blood cells/mm3), bile or intestinal contents is an indication for laparotomy. In about 60 per cent of cases, the mesenteric laceration is associated with a rupture of the intestine. If the tear is a large one and especially if it is transverse the blood supply to the neighbouring intestine is cut off and a limited resection of gut is imperative. Small wounds and wounds in the long axis should be sutured. If extensive damage to the mesenteric arcade of vessels is associated with damage to contiguous intestine, exteriorisation of the damaged segment is preferable to excision and suture.
Traumatic rupture
The intestine can be ruptured with or without an external wound — so-called blunt trauma. The most com-mon cause of this is a blow on the abdomen which crushes the bowel against the vertebral column or sacrum; also a rupture is more likely to occur where part of the gut has been fixed, for example, in a hernia, or where a fixed part of the gut joins a mobile one such as the duodenojejunal flexure. Here the damage may be retroperitoneal and easily overlooked.In small perforations the mucosa may prolapse through the hole and partly seal it, making the early signs misleading. In addition there may be a laceration in the mesentery. The patient will then have a combination of intra-abdominal bleeding and release of intestinal contents into the abdominal cavity, giving rise to peritonitis.
Traumatic rupture of the large intestine is much less common. In blast injuries of the abdomen following the detonation of a bomb, the pelvic colon is particularly at risk of rupture. Compressed air rupture can follow the dangerous practical joke where an air-line carrying compressed air is turned on near the victim’s anus.
Rupture of the upper rectum can occur during sigmoidoscopy and occasionally during the placement of rectal catheters for barium radiology.
Traumatic rupture of the colon can occur during colonoscopy. The most common site is the sigmoid colon where the formation of a sigmoid loop pushes against the antimesenteric border of the sigmoid colon, stretching it out and eventually perforating it.
Gun shot wounds and impalement injuries to the bowel have mote serious consequences because of the introduction of debris from the patient’s clothing or the missile itself mixing with the bacteria in the patient’s gut. High-velocity missiles may cause extensive damage of the bowel over a much wider area than just the entry and exit wounds.
Treatment
Where rupture is suspected a plain radiograph in the erect or lateral decubitus position will demonstrate the presence of free air in the peritoneal cavity or indeed in the retroperitoneal tissues. In almost all cases an abdominal exploration must be performed and, in many instances, simple closure of the perforation is all that is required. In others, for example, where the mesentery is lacerated and the bowel is not viable, resection may be necessary. In the case of the large intestine small clean tears can be closed primarily, if there is a large tear with damage to the surrounding structures to the adja-cent mesentery resection, exteriorisation may be used. Much depends on the amount of intra-abdominal soiling.
In the case of retroperitoneal portions of the intestine, for example, the duodenum, perforations can involve the front and back walls and the duodenum in particular has to be carefully mobilised to check that a concealed tear is not over-looked. In all cases the abdomen is washed out with saline and broad-spectrum intravenous antibiotics are given.
Bladder Trauma
Bladder injuries occur as a result of blunt or penetrating trauma. The probability of bladder injury varies according to the degree of bladder distention; therefore, a full bladder is more likely to become injured than an empty one.
Blunt trauma
Deceleration injuries usually produce both bladder trauma (perforation) and pelvic fractures.
Penetrating trauma
Assault from a gunshot or stabbing typifies penetrating trauma. Often, concomitant abdominal and/or pelvic organ injuries are present.
Obstetric trauma
During prolonged labor or a difficult forceps delivery, persistent pressure from the fetal head against the mother's pubis can lead to bladder necrosis.. Previous cesarean deliveries with resultant adhesions are a risk factor. Undue scarring may cause obliteration of normal tissue planes and facilitate an inadvertent extension of the incision into the bladder.
Gynecologic trauma
Bladder injury may occur during a vaginal or abdominal hysterectomy injury.
Urologic trauma
Perforation of the bladder during a bladder biopsy, cystolitholapaxy, transurethral resection of the prostate (TURP), or transurethral resection of a bladder tumor (TURBT) is not uncommon.
Orthopedic trauma
Orthopedic pins and screws can commonly perforate the urinary bladder, particularly during internal fixation of pelvic fractures
Traumatic bladder ruptures
Of traumatic ruptures, extraperitoneal bladder perforations account for 50-71%, intraperitoneal accounts for 25-43%, and combined perforations account for 7-14%. Incidence of intraperitoneal bladder ruptures is significantly higher in children because of the predominantly intra-abdominal location of the bladder prior to puberty.
Combined intraperitoneal and extraperitoneal ruptures account for approximately 10% of all traumatic bladder-perforating injuries. Mortality rates in these patients approach 60%, as compared to 17-22% overall, reflecting the severity of concomitant injuries associated with combined bladder
Pathophysiology
Bladder contusion is an incomplete or partial-thickness tear of the bladder mucosa. A segment of the bladder wall is bruised or contused, resulting in localized injury and hematoma. Contusion typically occurs in the following clinical situations:
Patients presenting with gross hematuria after blunt trauma and normal imaging studies
Patients presenting with gross hematuria after extreme physical activity (ie, long-distance running)
The bladder may appear normal or teardrop shaped on cystography. Bladder contusions are relatively benign, are the most common form of blunt bladder trauma, and are usually a diagnosis of exclusion. Bladder contusions are self-limiting and require no specific therapy, except for short-term bed rest until hematuria resolves. Persistent hematuria or unexplained lower abdominal pain requires further investigation.
Extraperitoneal bladder ruptures
Traumatic extraperitoneal ruptures usually are associated with pelvic fractures (89-100%). Previously, the mechanism of injury was believed to be from a direct perforation by a bony fragment or a disruption of the pelvic girdle. It is now generally agreed that the pelvic fracture is likely coincidental and that the bladder rupture is most often due to a direct burst injury or the shearing force of the deforming pelvic ring.
These ruptures usually are associated with fractures of the anterior pubic arch, and they may occur from a direct laceration of the bladder by the bony fragments of the osseous pelvis. The degree of bladder injury is directly related to the severity of the fracture.
With a more complex injury, the contrast material extends to the thigh, penis, perineum, or into the anterior abdominal wall. Extravasation will reach the scrotum when the superior fascia of the urogenital diaphragm or the urogenital diaphragm itself becomes disrupted.
Intraperitoneal bladder rupture
Classic intraperitoneal bladder ruptures are described as large horizontal tears in the dome of the bladder. The mechanism of injury is a sudden large increase in intravesical pressure in a full bladder. When full, the bladder's muscle fibers are widely separated and the entire bladder wall is relatively thin, offering relatively little resistance to perforation from sudden large changes in intravesical pressure.
Combination of intraperitoneal and extraperitoneal ruptures
Cystogram reveals contrast outlining the abdominal viscera and perivesical space. External penetrating injuries deserve special mention. A penetrating injury of the urinary bladder results from a high-velocity bullet traversing the bladder, knife wounds, or impalement by various sharp objects. These may result in intraperitoneal, extraperitoneal, or a combined bladder injury.
Clinical signs of bladder injury are relatively nonspecific; however, a triad of symptoms is often present (eg, gross hematuria, suprapubic pain or tenderness, difficulty or inability to void).
Most patients with bladder rupture complain of suprapubic or abdominal pain, and many can still void; Hematuria invariably accompanies all bladder injuries. Gross hematuria is the hallmark of a bladder rupture
An abdominal examination may reveal distention, guarding, or rebound tenderness. Absent bowel sounds and signs of peritoneal irritation indicate a possible intraperitoneal bladder rupture.
INDICATIONS
Foley catheter
Blood at the urethral meatus is an absolute indication for retrograde urethrography. Passage of a urethral catheter may convert a partially disrupted urethra into a complete tear.
CT scan
Cystogram The criterion standard for imaging a suspected bladder injury is a well-performed cystogram.
TREATMENT
Medical therapy
Most extraperitoneal ruptures can be managed safely with simple catheter drainage (ie, urethral or suprapubic). Leave the catheter in for 7-10 days, then obtain a cystogram. Approximately 85% of the time, the laceration is sealed and the catheter is removed for a voiding trial.
Virtually all extraperitoneal bladder injuries heal within 3 weeks. If the patient is taken to the operating room for associated injuries, extraperitoneal ruptures may be repaired concomitantly if the patient is stable.
Surgical therapy
Intraperitoneal bladder rupture
Most, if not all, intraperitoneal bladder ruptures require surgical exploration. These injuries do not heal with prolonged catheterization alone. Urine takes the path of least resistance and continues to leak into the abdominal cavity. This results in urinary ascites, abdominal distention, and electrolyte disturbances.
Surgically explore all gunshot wounds to the lower abdomen. Because of the nature of associated visceral injuries, immediately take patients with high-velocity missile trauma to the operating room, where the bladder injuries can be repaired concomitantly with other visceral injuries.
Stab wounds to the suprapubic area involving the urinary bladder are managed selectively. Surgically repair obvious intraperitoneal injuries, and manage small extraperitoneal injuries expectantly with catheter drainage.
Extraperitoneal extravasation
Bladders with extensive extraperitoneal extravasation often are repaired surgically. Early surgical intervention decreases the length of hospitalization and potential complications, while promoting early recovery.
Follow-up
Instruct the patient to return in 7-10 days for staple removal, and check the wound at that time.
Obtain the x-ray cystogram 10-14 days after surgery.
If the cystogram finding is normal, remove the urethral catheter.
Perform a voiding trial via the SPT.
Remove the SPT when the patient passes the voiding trial.
Advise the patient to return to normal activity within 4-6 weeks after surgery.
RENAL TRAUMA
Renal trauma may manifest in a dramatic fashion for both the patient and the clinician
Problem
Most renal trauma occurs as a result of blunt trauma. Renal injuries may be generally divided into 3 groups: renal laceration, renal contusion, and renal vascular injury. All subsets of renal trauma require a high index of clinical awareness and prompt evaluation and management.
Etiology
The mechanism of injury should alert the clinician to the possibility of renal trauma. The following list is not all-inclusive, but it highlights the major mechanisms that generate renal injuries.
Penetrating (eg, gunshot wounds, stab wounds)
Blunt (eg, pedestrian struck, motor vehicle crash, sports, fall)
Iatrogenic (eg, endourologic procedures, extracorporeal shock-wave lithotripsy, renal biopsy, percutaneous renal procedures)
Intraoperative (eg, diagnostic peritoneal lavage)
Other (eg, renal transplant rejection, childbirth [may cause spontaneous renal lacerations])
Clinical
The diagnosis of renal injury begins with a high index of clinical awareness. The mechanism of injury provides the framework for the clinical assessment. Particular attention should be paid to complaints of flank or abdominal pain. Urinalysis, both gross and, if necessary, microscopic, should be performed in patients who are thought to have renal trauma. Based on these initial measures, radiographic or operative investigation
Lab Studies
Urinalysis
Urinalysis provides rapidly available information in patients who may have a renal laceration; however, the data obtained must be viewed within a rational framework.
If gross hematuria is not present, a microscopic examination is advisable. Although a generalization exists that the degree of hematuria correlates with the likelihood of urinary tract trauma, renal injury with no hematuria has been reported.
Imaging Studies
Intravenous pyelogram
Computed tomography
Angiography
Ultrasonography
Diagnostic Procedures
Operative diagnosis
Depending on the mechanism of injury, many patients who sustain renal laceration have associated intra-abdominal injuries that require urgent exploration.
The clinical situation may have precluded the opportunity to perform the aforementioned diagnostic modalities.
The surgeon should be prepared to make the diagnosis of renal injury intraoperatively.
TREATMENT
Medical therapy
Nonoperative treatment
The kidney has an end artery blood supply with a segmental pattern of division that supplies the renal parenchyma. When subjected to blunt force that causes a laceration, the laceration tends to occur through the parenchyma. The resulting hematoma may displace renal tissue, but the segmental vessels themselves often are not lacerated. The closed retroperitoneal space around the kidney also promotes tamponade of bleeding renal injuries. Finally, the kidney is rich in tissue factor, the molecule that activates the extrinsic coagulation cascade, further promoting hemostasis after injury.
Interventional radiology has extended the ability to use a nonoperative approach.
Surgical therapy
Operative treatment
The goals of operative therapy for renal laceration incorporate the 2 basic principles of hemorrhage control and renal tissue preservation, which must be balanced for each individual patient.
At the time of the emergent laparotomy, the associated injury may be addressed. Evaluation and treatment of the renal injury is also possible. Patients with expanding hematomas or active hemorrhage should have their kidneys explored. Also, if the mechanism is penetrating trauma, most authors believe that the kidneys should be explored.
Patients with sound indications for emergent exploration include those with hemodynamic instability or missile injury to the abdomen. Unrelenting gross hematuria may require urgent exploration.
Operative technique can play a significant role in renal salvage.
Intraoperative details
Surgical techniques
Nephrectomy - Shattered kidney, multiple concurrent injuries, and uncontrolled hemorrhage
Partial nephrectomy - Avulsed fragments, polar penetrating mechanism, and collecting system repair
Adjuncts - Absorbable mesh wrap, topical thrombostatic agents, and omentum
Postoperative details
As with all trauma patients, the postoperative course should be monitored to ensure successful hemostasis. Serial hematocrit measurements should be considered. In patients in whom a damaged but perfused kidney is left in situ, renovascular hypertension remains a theoretical possibility and the patient should be monitored clinically for this entity.
STOMACH TRAUMA
CAUSES BY :
A ) The closed injuries are caused by blunt trauma like Blows, Falls , Sporting accidents , industrial injuries & Traffic accidents.They are either contusions or lacerations ( Ruptures). Contused area will be the seat of a delayed rupture. Rupture of the stomach will result in the gastric contents escaping into the peritoneal cavity resulting in chemical peritonitis. As the contents are relatively free of bacteria the signs and symptoms of peritonitis are delayed. Initially there is a local chemical peritonitis which will be followed by a bacterial general peritonitis.
B ) The open injuries are cause by penetrating Stab injuries , Missiles from firearms and Explosions. They may be found any where in the stomach and may involve both anterior and posterior walls. Unlike closed trauma their diagnosis is easy. Ruptures of the stomach are fatal in the ordinary course of nature
INVESTIGATIONS :
1 ) Chest X-RAY (look for the diaphragmatic injury , rip fracture near the stomach)
2 ) Erect and supine abdominal X-ray (can see any fracture in the stomach, any forigen body in the stomach , haematoma ,any displace in the abdominal region.)
3 ) Endoscopy can do .
4 )Intravenous urography , Contrast radiography , Arteriography , MRI , CT scans are occasionally indicated.
TREATMENT:
Patient should be hospitalisation
In close wounds with vicryl or PDS ( polydioxanone suture)
Pass nasogastric tube
LAPAROTOMY
Indications for laparotomy :
Eviscerations
Gunshot wounds (90 % have intra _abdominal damage )
Signs of spreading peritonitis
Subphrenic gas
Copious blood on peritoneal lavage
Continuing shock despite resuscitation.
Pancreatic Trauma
Etiology
Because of its anatomic position, an isolated pancreatic injury may occur with penetrating trauma to the mid back in the form of stab wounds or impalement. In a blunt trauma–induced isolated pancreatic injury, fracture over the spinal column is usually observed in smaller children and is caused by direct abdominal blows from malpositioned seat belts or intentional child abuse. Fortunately, both of these situations are relatively rare.
Usually, penetrating trauma caused by firearms results in the highest frequency of pancreatic injury and is almost always associated with concurrent injury to other intra-abdominal organs. This injury can result in a relatively simple isolated puncture of the body or tail of the pancreas (a highly complex and difficult injury) or an injury to the pancreatic head with involvement of the biliary and pancreatic ductal systems. In addition, the proximity of the larger vessels (eg, portal vein), the abdominal aorta, and the inferior vena cava (IVC) to the pancreatic head increases the risk of exsanguinating hemorrhage accompanying pancreatic penetrating injury. Exsanguinating hemorrhage due to concomitant vascular injury accounts for the greatest number of deaths in patients with pancreatic injury.
Clinical
The type of injury (ie, blunt vs penetrating) and information about the injuring agent (eg, GSW, knife) help focus the clinician on the possibility of pancreatic injury.
During the physical examination, seat belt marks, flank ecchymoses, or penetrating injuries should alert the physician to the potential for pancreatic injury. Pancreatic injury can be frighteningly symptom free early in the postinjury time frame and even silent in many cases. Rarely, a contained fracture of the spleen with retroperitoneal hematoma or leak manifests as dull epigastric pain or back pain, but the more common scenario is for patients to exhibit severe peritoneal irritation and a positive abdominal examination finding, usually caused by injury to other organs. Symptoms of injury to other structures commonly mask or supersede that of pancreatic injury, both early and late in the hospital course. Therefore, a high degree of clinical awareness is necessary to ensure that pancreatic injuries are not overlooked or missed, either early in the course of trauma or later in the ICU when the patient is not clinically improving as expected.
Lap diagnosis
Plain films of the abdomen may show pancreatic calcification from previous episodes of pancreatitis but are rarely of any benefit in detecting blunt trauma. These films can be valuable in detecting penetrating trauma by visualizing and localizing foreign bodies such as bullet fragments and projectile-induced bony injury. Frequently, these films can be obtained simultaneously with the bony pelvis films advocated by advanced trauma life support (ATLS) protocols in trauma victims by use of a larger x-ray plate and widening of the field of x-ray exposure.
Kidney, ureter, bladder (KUB) film or upright abdominal films rarely provide useful information and only serve to delay the implementation of further care or diagnostic measures.
While not specifically useful in the detection of pancreatic trauma, the upright chest film may show free air under the diaphragm, which is suggestive of an associated gastric, duodenal, or small bowel injury and is frequently associated with pancreatic injury.
CT scan
In a patient who is hemodynamically stable, a CT scan provides the safest and most comprehensive means of diagnosis of pancreatic injury. Unfortunately, the sensitivity of this modality is reported only to be in the range of 40-68%, so patients must be monitored closely if pancreatic injury is suspected. Most reports have examined single detector or spiral scanners, but 64 detector helical scanners that are now available may provide a more accurate determination of injury. A CT scan may be augmented by the judicious use of ERCP in select cases. Laparotomy has a higher sensitivity but is not a reasonable screening test for all suspected cases of pancreatic injury.
A CT scan of the abdomen provides the simplest and least invasive diagnostic modality currently available to aid in the detection of a stable blunt pancreatic injury. However, this study is only rarely useful in acute penetrating injury.
A workup for patients who are stable and have knife wounds to the back or flank may include a CT scan, but a patient who is unstable must never be placed in the CT scanner, whether the injury is blunt or penetrating trauma.
CT scan is contraindicated in patients who are hemodynamically unstable or who have a penetrating trauma in which the decision for operative intervention has been made.
A CT scan of the pancreas is also useful in the follow-up care of patients with a pancreatic injury and trauma. Traumatic pancreatic cysts, pseudocysts, delayed ductal injury, pancreatic transection, pancreatitis, abscess, pancreatic necrosis, and splenic artery aneurysms may be noted after surgery or after the patient is released from the hospital.
Magnetic resonance cholangiopancreatography (MRCP) is being used more frequently in Level 1 Trauma Centers to assess injury to the ductal components but has not been prospectively compared to CT or other modalities.
Other Tests
Intraoperative cholangiograms and pancreatic ductograms, especially with reflux into the pancreatic ducts (eg, Wirsung, Santorini), frequently provide information regarding the status of the injured pancreas when direct visualization is not helpful. Some authors recommend that these studies be performed during operative exploration, noting that they may help decrease complications due to missed pancreatic ductal injuries.
Diagnostic Procedures
In the patient who is unstable, operative exploration provides the optimal diagnostic tool for pancreatic injury. As in blunt trauma, endoscopic retrograde cholangiopancreatography (ERCP) or intraoperative dye studies may provide more information in a select patient population.
ERCP is increasingly being used to help diagnose, both immediately and in delayed fashion, the presence of pancreatic ductal injuries. Some authors suggest early ERCP (ie, within 6-12 h of injury) to minimize delayed complications. While extremely helpful, this procedure has potential complications that can limit its usefulness in patients with pancreatic trauma. For it to be of benefit, the endoscopist must be skilled and experienced in its use in the injured and potentially severely ill trauma patient. This is especially true when used in the operating room in a patient with an open abdomen who is at risk for hypothermia with exposed abdominal contents.
Histological Findings
Histological examination of the resected pancreas documents the presence of hemorrhage and, frequently, of crush injuries to the tissue. Occasionally, this examination may reveal chronic preexisting pancreatic conditions such as pancreatitis, saponification, scarring, or tumors.
Staging
Visit the American Association for the Surgery of Trauma (AAST) Web site for a published injury scoring system for pancreatic trauma that correlates to morbidity and mortality.
TREATMENT
Medical therapy
In the early 1900s, observation of pancreatic injury was associated with a 100% mortality rate. However, more recently, the medical literature supports observation in select blunt injuries to the pancreas. The standard of care in penetrating injuries is still operative exploration.
Patients who have experienced blunt trauma and who have stable hemodynamics and CT scans showing no evidence of pancreatic parenchymal fracture, parenchymal hematoma, parenchymal edema, fluid in the lesser sac, or retroperitoneal hematoma may be observed but should not be considered to be cleared for pancreatic injury for at least 72 hours. Any patient with blunt trauma who continues to have abdominal pain or who develops symptoms of pancreatic injury should be thoroughly reassessed for pancreatic injury and operative intervention.
Surgical therapy
Surgery is by far the most common therapeutic modality for patients with pancreatic trauma, especially in those with penetrating trauma, in whom exploratory laparotomy is both a diagnostic and therapeutic measure. Vasquez et al (2001) showed improved outcomes when penetrating pancreatic injury therapy was based on injury grade and location.
In most cases of blunt injury, ductal damage can be visualized directly. Most commonly, the damage is minor and such findings as capsular tears, superficial lacerations, bullet wounds of the body or tail, small contusions, or hematomas should be visualized and documented; however, they should not be surgically explored unless ductal injury is suspected. Soft closed suction drains (eg, Jackson-Pratt, Blake) should be used. The author usually uses 2 drains, but a single drain may suffice in some situations. Continued drainage with high amylase levels persisting beyond 48-72 hours is highly suggestive of a missed ductal injury. These problems must be treated with workup of the ductal integrity with ERCP or another modality and may require another operation. Occasionally, a trial of total parenteral nutrition (TPN) or elemental diet through a feeding jejunostomy may result in decreased drainage and closure of the leak.
Significant blunt trauma to the pancreas with parenchymal fracture is easily visualized on exploration by noting the retroperitoneal hematoma around the pancreas at the spinal column. Patients with ductal injury usually require resection of the tail and body distal to the vertebral column, and patients with documented intact ductal systems may be drained and observed.
More details regarding pancreatic surgical technique can be found in the Intraoperative details section below. Combined pancreatic and duodenal injuries are not reviewed in this article.
Preoperative details
Adherence to ATLS standards and adequate preparation for emergency surgery in patients with pancreatic injury reduces morbidity and mortality rates. Early surgical intervention with identification of ductal injuries has been shown to reduce the incidence of early and late complications and death.
Intraoperative details
Blunt injury
In most cases of blunt injury, surgical resection is not necessary. Small or superficial capsular tears, contusions, or hematomas are best managed without sutures. Wide drainage with soft closed suction drains suffices in 80-90% of patients with pancreatic injuries. Pancreatic parenchymal transection against the vertebral bodies may require resection of the body with oversewing of the distal duct with a nonabsorbable suture and drainage of the pancreatic bed. If possible, the distal parenchyma should also be oversewed, but contused, edematous pancreatic parenchyma is notoriously difficult to sew, and drainage of the bed may be all that is possible in this situation. Some surgeons use linear surgical staplers, which create a staple line that is 1.5 mm in depth.
Make sure to assess and suture ligate the pancreatic duct in these cases. Ligation of the duct has traditionally been performed with a nonabsorbable suture, but a few authors have had good results with the newer, long-lasting monofilament absorbable sutures.
Splenic preservation, although ideal, is frequently not possible with a fracture of the pancreatic body. The same anatomic orientation over the spinal column that created the parenchymal fracture and ductal injury has usually caused a splenic artery or venous injury, which results in thrombosis or aneurysmal formation and eventual splenic loss. Resection of the pancreas at the vertebral column usually leaves 40-50% of the glandular tissue, so permanent diabetes and exocrine insufficiency are unusual after resection.
Penetrating trauma
As with blunt trauma, examination and peripancreatic drainage is the most common intervention, but the range and severity of penetrating injuries to the pancreas are much more extreme. Resection of the tail or body is accomplished in a similar fashion and is technically simple, but injuries to the head and neck of the pancreas may require more creative and more difficult operative therapies.
Penetrating trauma to the head and neck of the pancreas without ductal injury can be managed with simple drainage. The appearance of bile from a penetrating injury should alert the surgeon to the possibility of a ductal injury, and a cholangiogram or ductogram is extremely valuable in helping the surgeon to plan the needed operative intervention. Isolated minor ductal damage can occasionally be stented operatively or by the interventional radiologist and should always be accompanied by an exploratory laparotomy with wide local drainage and close observation.
For patients in whom the duodenum is intact but injury to the pancreatic head and ductal system precludes repair, stenting, or local drainage, some authors suggest a Roux-en-Y pancreaticojejunostomy to preserve the pancreatic parenchyma. While theoretically feasible, the actual incidence of its use is rare (summarized in separate reviews by Graham et al and Jones). Asenio et al provide medical illustrations of many of the rarely used surgical options for trauma to the head and neck of the pancreas in the monograph on management of pancreatic injuries.
Fortunately, massive injury to the head of the pancreas, including the duodenum, rarely requires a trauma Whipple operation or pancreaticoduodenectomy. The mortality rate remains high in these cases, with even experienced centers reporting only a 50-64% survival rate. Unless the patient is actively exsanguinating, surgeons in smaller facilities with limited operating and intensive care unit (ICU) facilities should consider damage-control methods, stabilize injuries, staunch active hemorrhage, and then transfer the patient to the closest level 1 trauma center, where experience with this type of injury is more common.
The intimate anatomic position of the vena cava, portal vein, and mesenteric artery and vein with the pancreas also creates significant problems. Higher mortality rates in these cases are caused by uncontrolled hemorrhage rather than pancreatic injury; make every effort to repair these injuries first, directing attention to the pancreatic injury only after vascular integrity has been accomplished. Damage-control techniques may be necessary in these situations, addressing the pancreatic injury at a second operation, 12-48 hours later, when the patient has been warmed and stabilized.
Postoperative details
In the recovery room, direct attention toward warming the patient; monitoring metabolic acidosis, especially in prolonged operations; and maintaining normal hemodynamic parameters. Adequate urine output, vigorous intravenous fluid replacement with crystalloid solution and blood products (as needed), and mechanical support of ventilation are necessary.
The second greatest cause of death related to pancreatic injury is noted in the ICU during the postoperative period. As might be expected, death is most common with massive injury of multiple organs and a history of significant blood loss. Acute respiratory distress syndrome (ARDS), multisystem organ failure, and infection are the most common causes of delayed death in these situations. In cases of more isolated pancreatic injury, common early complications include fistula formation, pancreatitis, and abscess formation.
COMPLICATIONS
Complications of pancreatic injury are myriad and run the gamut from minor pancreatitis prolonging the hospital stay to death.
Fistula formation is the most frequently reported complication, but with wide local drainage and good nutrition and supportive care, fistulas usually resolve spontaneously within 2 weeks of injury. Prolonged output of greater than 250 mL/d for more than 2 weeks or outputs of 750 mL/d or more should prompt ERCP or other diagnostic evaluation of the ductal system. Somatostatin analogues have been reported anecdotally in multiple prospective randomized clinical trials to decrease fistula output and to facilitate closure, but they have not been proven to be of absolute benefit. The commercially available analogue is expensive and, if used, should be closely monitored for its effects in individual patients.
Delayed diagnosis and delayed surgery while under observation show a higher rate of pancreas-specific morbidity and mortality. Early diagnosis and therapy are associated with better overall outcomes in these patients.
More recent papers in the trauma literature have reported an increase in infectious complications in patients when pancreatic wounds occur in conjunction with hollow viscus injury (ie, duodenum, small bowel, colon). While the pathophysiology of this finding has not been well elucidated yet, experimental data in rats with pancreatic ductal ligation protected from hemorrhagic shock induced lung injury and, to a lesser degree, gut injury. Pancreatic proteases may activate digestive enzymes in ischemic intestine, which then travel via lymphatics to cause lung injury.
Delayed complications of recurrent pancreatitis, pancreatic pseudocysts, splenic artery aneurysm, and endocrine or exocrine insufficiency have also been reported
OUTCOME AND PROGNOSIS
Outcome from minor and isolated pancreatic injury is usually quite good. Complications are rare, and functional outcome is excellent.
Outcome from severe pancreatic injury is much poorer overall than with other organ injuries. This outcome is primarily due to the frequent presence of associated life-threatening injuries and the unforgiving nature of the pancreas, both for missed injury and after major emergency operative intervention.
ГОУ ВПО «Смоленская государственная медицинская академия
Федерального агентства по здравоохранению и социальному развитию»
МЕТОДИЧЕСКИЕ УКАЗАНИЯ ДЛЯ СТУДЕНТОВ
ПО ДИСЦИПЛИНЕ хирургические болезни
SURGERY OF CHEST TRAUMA
Составитель ассистент А.Л.Буянов
Методические указания утверждены на методическом совещании кафедры госпитальной хирургии (протокол № 2 от 6 октября 2008 г.)
Зав. кафедрой______________(проф. С.А.Касумьян)
2008 г.
Many of the surgically treatable conditions of the lung are treated at specialist thoracic centres and the only exposure to thoracic surgery that most surgeons have is dealing with thoracic trauma. The approach to treatment must be methodical and exact because the signs, particularly in the presence of other injury, may easily be missed. The guidelines produced by the American College of Surgeons Advanced Trauma Life Support (ATLS) Group provide a thorough and unambiguous approach to trauma. The general principles of resuscitation and need for priorities are extensively discussed and will not be repeated here. However, the specific aspects of trauma management related to the thorax will be covered. Thoracic trauma is responsible for over 70 per cent of all deaths following road traffic accidents. Blunt trauma to the chest in isolation is fatal in 10 per cent of cases, rising to 30 per cent if other injuries are present. An increasing number of penetrating thoracic wounds are also seen from domestic and civil violence and have a mortality rate of 3 per cent for simple stabbing to 15 per cent for gunshot wounds.
Initial management
Early deaths after thoracic trauma are caused by hypoxaemia, hypovolaemia and tamponade. The first steps in treating these patients should be to diagnose and treat these problems as early as possible because they may be readily corrected. Young patients have a large physiological reserve and serious injury may be overlooked until this reserve is used up; then the situation is critical and may be irretrievable. The best approach is to maintain a high index of suspicion and suspect the worst if life-threatening conditions are to be avoided. Early consultation with a regional thoracic centre is advised in cases of doubt. In an emergency it is essential that experienced help is summoned immediately.
The basic principles of resuscitation are securing the airway and restoring the circulating volume. Blood and secretions are removed from the oropharynx by suction. If the patient is unable to maintain his or her airway then an oropharyngeal airway followed by tracheal intubation (once a cervical spine injury is excluded) may be necessary.
A thorough inspection of the chest wall includes noting the frequency and pattern of breathing, external evidence of trauma and structural defects of the thorax. Palpation will detect surgical emphysema, paradoxical movement and a stove-in chest. Auscultation and percussion should reveal the existence of a pneumothorax (there is decreased movement on the affected side with a hyperresonant percussion note and shift of the trachea to the opposite side) which requires emergency drainage (see Pneumothorax for a more detailed appraisal and see Chest drainage for advice on technique of drain insertion).
Once the patient has been stabilised then radiographs of the chest should be taken and further treatment decided on the basis of the patient's condition and the radiographic result. It is rarely necessary to perform a thoracotomy in the resuscitation room but, in the case of tamponade from a penetrating injury it might be life saving. However, the fact is that, even in experienced hands, the yield in terms of survival in this group of patients is very small. If there is profound hypotension as a result of cardiac tamponade, needle aspiration of the pericardium is life saving and may hold the situation long enough for more controlled surgery to be performed.
The components of chest injury in blunt trauma
Any combination of structures may be involved in varying degrees of severity. If the skeletal injuries are severe, underlying parenchymal injuries are likely to be in proportion; however, in young flexible chests, or those restrained by seat belts, there may be little external evidence of the severity of internal damage.
Chest wall. Localised rib fracture due to direct trauma. A simple rib fracture may be serious in elderly people or those with chronic lung disease who have little pulmonary reserve. Uncomplicated fractures require sufficient analgesia to encourage a normal respiratory pattern and effective coughing. Oral analgesia may suffice but intercostal nerve blockade with local anaesthesia may be very helpful. Chest strapping or bed rest is no longer advised and early ambulation with vigorous physiotherapy (and oral antibiotics if necessary) is encouraged. A chest radiograph is always taken to exclude an underlying pneumothorax. It is useful to confirm the skeletal injuries but routine chest radiography may miss rib fractures. However, once a pneumothorax and major skeletal injuries are excluded, the management is the same - the local control of chest pain.
Major chest wall trauma
Flail chest (Fig. 1). This occurs when several adjacent ribs are fractured in two places either on one side of the chest (or either side of the sternum). The flail segment moves paradoxically, that is, inwards during inspiration and outwards during expiration, thereby reducing effective gas exchange. The net result is poor oxygenation from injury to the underlying lung parenchyma and paradoxical movement of the flail segment. The underlying lung injury with loss of
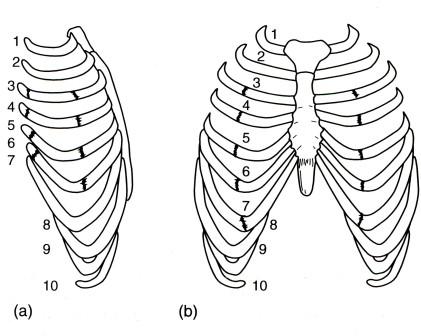
Fig. 1. Diagram of flail chest: (a) lateral flail;
(b) anterior flail ('stove-in' chest).
alveolar function may result in deoxygenated blood passing into the systemic circulation. This creates a right-to-left shunt and prevents full saturation of arterial blood. In the absence of any other injuries and, if the segment is small and not embarrassing respiration, the patient may be nursed on a high dependency unit with regular blood gas analysis and good analgesia until the flail segment stabilises. In the more severe case, endotracheal intubation is required with positive pressure ventilation for up to 3 weeks, until the fractures become less mobile. Thoracotomy with fracture fixation is occasionally appropriate if there is an underlying lung injury to be treated at the same time. An anterior flail segment with the sternum moving paradoxically with respiration can be stabilised by internal fixation but operative management is not usual for either.
First rib fracture. Fracture of the first rib should alert the clinician to a potentially serious chest injury. This rib is well protected and requires a considerable force to fracture and associated injuries to the great vessels, abdomen, head and neck are common. The mortality rate associated with a fracture of the first rib exceeds 30 per cent. Similar suspicions are raised when fractures of the sternum and scapula are seen. Fractures of the lower ribs may involve underlying abdominal viscera (spleen on the left and liver on the right). Intercostal artery bleeding may still be severe, resulting in haemothorax.
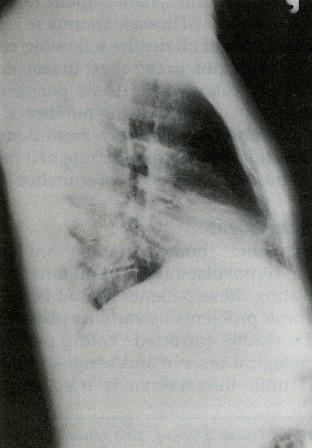 Fractures
of the sternum (Fig.
2). This injury is now seen as a result of deceleration on to seat
belts. Steering wheel injuries are now much less common. The injury
is very painful even in the mild case where only the external plate
of the sternum is fractured. However, there is a real risk of
underlying myocardial
Fractures
of the sternum (Fig.
2). This injury is now seen as a result of deceleration on to seat
belts. Steering wheel injuries are now much less common. The injury
is very painful even in the mild case where only the external plate
of the sternum is fractured. However, there is a real risk of
underlying myocardial
Fig. 2. Radiograph of sternal fracture.
damage and the patient should be observed in hospital with constant ECG monitoring, analgesia and serial cardiac enzymes. Rupture of the aorta and associated cervical spine injuries also need to be excluded. Most cases need no specific treatment but paradoxical movement or instability of the chest may need more active management. It should be remembered that sternal fracture may occur during closed cardiac massage.
Vertebrae
The thoracic spine may be injured as one component of multiple injury or in isolation. It is more usual for the cervical spine to be injured and this must be excluded before any manipulations or movements take place. Damage to the thoracic spine is likely to be associated with injuries to other thoracic viscera. The assessment and treatment of spinal injury are discussed in Chapter 26. However, the thoracic spine injury is a reminder that, in patients where the chest injury predominates, a quick screening neurological examination confirming the integrity of the nerve supply to the lower limbs should be performed and documented.
Pleura
If the visceral pleura is breached (most commonly by a rib fracture) pneumothorax follows. Generation of positive pressure in the airways by coughing, straining, groaning or positive pressure ventilation will result in tension pneumothorax. The pleural space may also fill with blood as a result of injury anywhere in its vicinity. Remember that an erect chest radiograph is the only sure way to confirm or exclude the diagnosis of pneumothorax and should be obtained if at all possible. Early management of tension pneumothorax is life saving. Also, good management of the pleural space pre-empts many later complications from clotted haemothorax, constriction of the lung and empyema. The diagnosis and management of a simple pneumothorax are discussed in detail later, but in the trauma situation it is imperative to consider tension pneumothorax and haemothorax.
Traumatic pneumothorax. Blunt trauma to the chest wall may result in a lung laceration from a rib fracture. All traumatic pneumothoraces require drainage through an underwater seal drain because of the possibility that they may become a tension pneumothorax with mediastinal shift and circulatory collapse. There is decreased air entry on the affected side and the trachea may be pushed over to the opposite side. There is an increased percussion note. If a tension pneumothorax is suspected on clinical grounds, treatment is necessary before radiographs can be taken. A wide-bore needle introduced into the affected hemi-thorax will release any air under tension and is life saving. A wide-bore intercostal tube is introduced laterally and directed to the apex of the pleural cavity. A second drain may be introduced basally to drain blood.
Chest drain insertion
Insertion of a chest drain is indicated when there is air or fluid in the pleural cavity (Fig. 3). The site of insertion is in the triangle of safety. The area chosen is infiltrated with local anaesthetic and an incision is made in the skin and subcutaneous tissues, sufficient to admit a finger easily. The intercostal muscles are separated with artery forceps and the pleura punctured. The intercostal drainage tube is inserted with the stylette withdrawn, so as not to damage the underlying lung tissue. A large-bore tube is used for the drainage of blood and fluids, whereas a smaller-bore tube may be used for the removal of air.
Traumatic haemothorax. Drainage is essential because re-expansion of the lacerated lung compresses the torn vessels and reduces further blood loss. Drainage will also allow the mediastinal structures to return to the midline and relieve compression of the contralateral lung. If left, a dense fibrothorax will result with the possibility of an added empyema. The procedure is similar to drainage for pneumothorax but a wide bore tube (>28 Fr) is required and a basal drain is sometimes necessary. Continuing blood loss in excess of 200 ml/hour may require urgent thoracotomy within the first few hours.
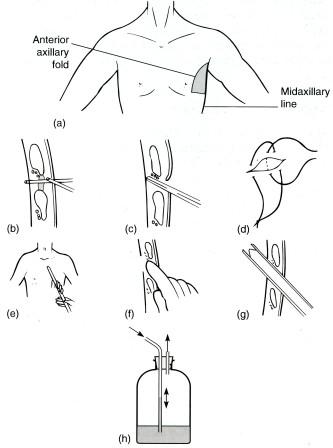
Fig. 3. Insertion of chest drain, (a) Triangle of safety; (b) penetration of the skin, muscle and pleura; (c) blunt dissection of the parietal pleura; (d) suture placement; (e) gauging the distance of insertion; (f) digital examination along the tract into the pleural space; (g) withdrawal of central trochar and positioning of drain; (h) underwater seal chest drain bottle.
Lung parenchyma
Lung contusion. The underlying lung is often injured in moderate-to-severe blunt thoracic trauma and the area of contusion may be extensive. This usually resolves but lacerations with persistent air leak may require exploration by thoracotomy. It is important to prevent infection of the underlying lung by early mobilisation (if the patient's condition permits), prophylactic antibiotics, suction drainage and physiotherapy. The importance of a good quality postero-anterior erect chest radiograph following any trauma to the lung cannot be overemphasised (Fig. 4).
Major airways
Injuries to major bronchi are infrequently seen as the patient rarely survives the insult leading to major airway disruption. There is usually a combination of surgical emphysema, haemoptysis and pneumothorax. Chest drainage in spite of the addition of suction fails to reinflate the lung and a persistent air leak may be present. Injury to the trachea requires considerable force and consequently less than a quarter of patients survive to reach hospital. The injury may be from direct trauma or the result of high intratracheal pressure against a closed glottis. There is hoarseness, dyspnoea and surgical emphysema. The exact pattern of signs will depend on the site of the injury and whether or not the pleura has been breached. The treatment is exploration and repair if possible. Resection of lung should be avoided as a surprising degree of recovery may occur.
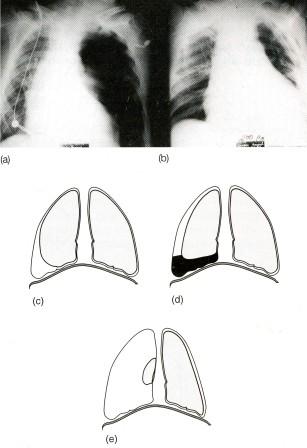
Fig.4.
(a) Traumatic tension pneumothorax showing mediastinal shift and compression of the contralateral lung;
(b) resolution of the radiographic appearances following insertion of intercostal tube. Diagrammatic appearances of haemothorax:
(c) minor pneumothorax;
(d) haemopneumothorax;
and (e) tension pneumothorax.
Diaphragm
Diaphragmatic rupture. The mechanism for diaphragmatic rupture is high-speed blunt abdominal trauma with a closed glottis. The sudden rise in intra-abdominal pressure breaches the weakest part of the abdominal wall, namely the diaphragm. This occurs much more commonly on the left hemidiaphragm (the right is protected by the liver). Colon and stomach may herniate into the thorax displacing the lung. Bowel sounds may be heard in the chest and the chest radiograph may reveal bowel gas in the lung fields. A contrast study will confirm the diagnosis. Occasionally, the injury is overlooked and the patient presents some time later with a diaphragmatic hernia. Cases presenting acutely should be explored by thoracotomy not only to repair the diaphragm and prevent respiratory embarrassment, but to exclude injury to an underlying abdominal viscus such as the spleen. Penetrating injuries below the level of the eighth rib may penetrate the diaphragm and injure an underlying abdominal viscus.
Cardiac injury
Major injuries to the heart and great vessels from blunt trauma are frequently fatal and the patient rarely survives long enough to reach hospital. The injuries that are encountered in the accident and emergency department are the following.
Myocardial contusion. This must be suspected when the sternum is fractured although the true incidence is not known. Myocardial damage from trauma will give an ECG pattern similar to myocardial infarction and enzyme changes may occur. In severe trauma there may be arrhythmias and signs of heart failure. Patients with ECG changes and enzyme rises even in the absence of any problems should be nursed in a high-dependence areа with full monitoring and resuscitation equipment available. There is no specific treatment in the uncomplicated case but the risk of fatal arrhythmia diminishes after 48 hours or until the enzymes have returned to normal and any ECG changes have resolved. Occlusion of the coronary arteries progressing to discrete, localised myocardial infarction has been documented.
Chamber rupture and valve blow-out. This is well described and is thought to occur if the ventricle is compressed just before systole at the point of maximal diastolic filling. Chamber rupture is likely to be fatal and those that do survive are likely to have an atrial rupture. Rupture of the mitral or tricuspid valve may not be immediately apparent, but a loud pansystolic murmur should arouse suspicion. Surgical treatment usually results in dramatic improvement in these patients.
Aorta
Aortic transection (Fig. 5). This is usually the result of a major deceleration injury (road traffic accident or a fall from a height) and the patient often has other injuries. However, only about 15 per cent of patients with aortic transection survive long enough to reach hospital. Of these, two-thirds would die of late rupture within 14 days and the remainder would be at risk from rupture of a developing chronic false aneurysm. As the site is remarkably consistent, presumably it is the anatomy of the aorta which determines the site of rupture. The vessel is relatively fixed at the site of the ligamentum arteriosum, just distal to the left subclavian artery, and from there down is tethered to the vertebral column by intercostal arteries and mediastinal pleura. The shear forces from a sudden impact disrupt the intima and media resulting in retraction. If the intima is not breached, the patient may be stable but the development of a left-sided pleural effusion is an ominous sign. Transection of the aorta must be suspected in all high-speed deceleration incidents. The clinical signs may be masked by concurrent injury but include intrascapular pain, a murmur, hoarseness and radiofemoral delay of the arterial pulse. The condition may be completely asymptomatic. A good quality posteroanterior chest radiograph showing widening of the mediastinum is very suspicious. Portable chest radiographs taken from the front (anteroposterior) always magnify the mediastinal shadow and cause uncertainty. Aortography is the diagnostic investigation and should be available at most hospitals. Computed tomography is unhelpful and misleading because transections have little length and intimal lesions are missed on 1-cm slices (in marked contrast to aortic dissection where computed tomography is a valuable investigation).
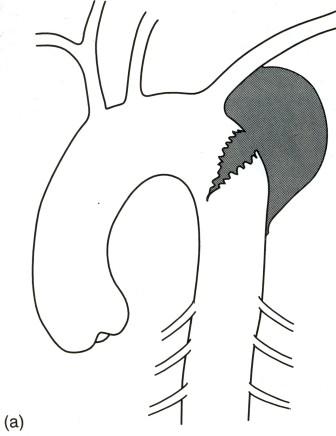
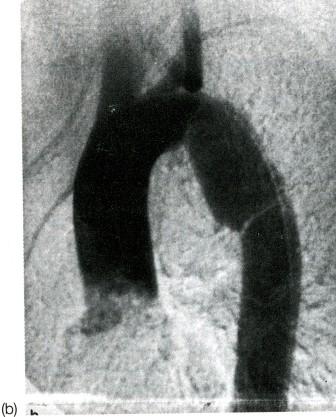
Fig. 5 (a) Diagram of aortic transection; (b) aortogram of transection. ((b) Reproduced from Unsworth-White J., Kallis P., Treasure T. Thoracic trauma. Surgery 1993; 11:3:356 by kind permission of The Medicine Group (Journals) Ltd.)
O nce the diagnosis is made the treatment is urgent exploration via a left thoracotomy through the fourth intercostal space. Control above and below the transection is vital and the aorta is repaired by direct suture or interposition graft (Fig. 6). There is a risk of paraplegia (15 per cent) with this procedure and this should be specifically mentioned to the patient and the relatives before surgery. A heparin-bonded shunt (Gott) to maintain lower body perfusion may reduce the risk of paraplegia. Cardiopulmonary bypass is unsafe because systemic heparinisation is required in an already multiply injured patient.
Fig. 6. Diagram of repair of transection
Management of blunt chest trauma
Most chest injuries where the heart is not injured are managed conservatively with underwater seal drainage if necessary, and oxygen and physiotherapy to help the patient expectorate while the underlying lung parenchyma heals. In about 10 per cent of cases a thoracotomy is required. The indications for thoraco-tomy following blunt thoracic trauma are the following:
• 500-1000 ml of blood at the time of initial drainage is common and may need no further action but greater volumes, especially if the blood is fresh, require intervention.
• Continued brisk bleeding (>100 ml/15 min) from the intercostal drains indicates a serious breach of the lung parenchyma and urgent exploration is required.
• Continued bleeding of >200 ml/h for 3 or more hours may require thoracotomy under controlled conditions.
• Rupture of the bronchus, aorta, oesophagus or diaphragm.
• Cardiac tamponade (if needle aspiration is unsuccessful).
All explorations following trauma should have double-lumen tube endotracheal intubation to facilitate surgery on the injured side and to protect the undamaged lung.
If transfer is undertaken, the patient must be stabilised before the journey. All lines must be secured and ECG monitoring available. Chest drains must not be clamped during transfer and a medically qualified person should accompany the patient.
Penetrating injury
In some aspects, penetrating thoracic injury is simpler to deal with than blunt trauma because the wound is visible and the structures at risk can be quickly assessed. A defect in the chest wall through to the pleura is a 'sucking wound7. The underlying lung collapses and air moves in and out of the thorax with each breath. Emergency treatment involves sealing the wound and intercostal drainage. Definitive treatment may then follow. It is important to establish the path or track of bullet and stab wounds in the chest as there may be damage to the heart, great vessels, and the diaphragm and abdominal viscera in addition to the lung injury.
They are bringing him down,
He looks at me wanly.
The bandages are brown,
Brown with mud, red only -
But how deep a red in the breast of the shirt,
Deepening red too, as each whistling breath
Is drawn with the suck of a slow filling squirt
While waxen cheeks waste to the pallor of death.
From Casualty, 1917
By Robert Nichols 1893-1944,
one of the First World War poets
Bullet wounds create a cavitating defect in the tissues that they pass through. The tissue damage may be very extensive with high-velocity missiles and entry and exit wounds should be noted. Lung tissue is more compliant than the bone and muscles that comprise the limbs, and enthusiastic resection along the track can be avoided in most cases. Tetanus prophylaxis and high-dose antibiotics (to cover anaerobic organisms) should be given. Bullets lodged in the lung do not require removal if they are not causing any problems.
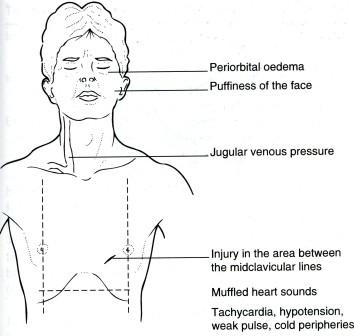 Penetrating
wound of the heart. This
is usually the result of a stabbing or shooting incident, but can
also be iatrogenic from central line placement, cardiac
catheterisation and endomyocardial biopsy. Cardiac tamponade may
occur rapidly even with small amounts of blood in the pericardium and
the condition is recognised by low blood pressure, tachycardia, a
high central venous pressure, pulsus paradoxus and faint heart sound
(Fig. 7). Emergency treatment includes aspiration of the pericardium
by advancing a wide-bore needle to the left of the xiphisternum
towards the heart. This may hold the situation until surgical repair
is performed. The heart is exposed via a median sternotomy with
incision of the pericardium in the midline. For the more generally
trained surgeon or those without the necessary equipment to saw the
sternum, a left anterior thoracotomy may be preferred. The
pericardial cavity is evacuated and the cardiac defect repaired using
buttressed sutures. Bullets in cardiac chambers should be removed
under cardio-pulmonary bypass.
Penetrating
wound of the heart. This
is usually the result of a stabbing or shooting incident, but can
also be iatrogenic from central line placement, cardiac
catheterisation and endomyocardial biopsy. Cardiac tamponade may
occur rapidly even with small amounts of blood in the pericardium and
the condition is recognised by low blood pressure, tachycardia, a
high central venous pressure, pulsus paradoxus and faint heart sound
(Fig. 7). Emergency treatment includes aspiration of the pericardium
by advancing a wide-bore needle to the left of the xiphisternum
towards the heart. This may hold the situation until surgical repair
is performed. The heart is exposed via a median sternotomy with
incision of the pericardium in the midline. For the more generally
trained surgeon or those without the necessary equipment to saw the
sternum, a left anterior thoracotomy may be preferred. The
pericardial cavity is evacuated and the cardiac defect repaired using
buttressed sutures. Bullets in cardiac chambers should be removed
under cardio-pulmonary bypass.
Fig. 7. Diagram for suspicion of cardiac injury.
How to do a thoracotomy
All surgeons dealing with trauma victims should be able to perform a thoracotomy if required. The standard route into the thoracic cavity is through a posterolateral thoracotomy. The incision is used for access to the following:
• The lung and major bronchi.
• The thoracic aorta (aneurysm resection, repair of transection, coarctation repair and ligation of patent ductus arteriosus).
• The oesophagus (resection and repair).
• The posterior mediastinum (for mediastinal mass resection).
Following induction of anaesthesia, a preoperative rigid bronchoscopy is performed, especially if a resection for cancer is contemplated. A double-lumen tube is used to control the lungs separately, if desired. Ventilation may be maintained by ventilating one lung while the other is collapsed to facilitate surgery. However, remember that they were devised to protect the underlying lung from pus and blood, and to retain under the anaesthetist's control. The patient is turned on to the unaffected side in the lateral position (Fig. 8). The lower leg is flexed at the hip and the knee with a pillow between the legs. Table supports are used to maintain the position and additional strapping is used at the hips for stability. The upper arm is supported by a bracket in a position of 90° flexion. The lower arm is flexed and positioned by the head. It is important for the surgeon to be completely satisfied with the position of the patient at this stage.
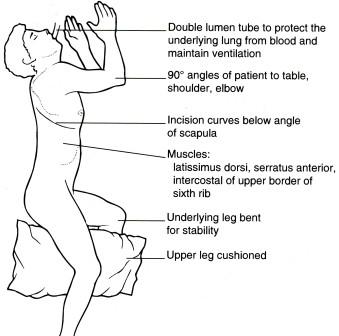
Fig. 8. Diagram of correct positioning for thoracotomy.
The incision is made from 1 to 2 cm inferolateral to the nipple in men and the inframammary fold in women. The incision extends along 1-2 cm below the tip of the scapula and extends posteriorly and superiorly between the medial border of the scapula and the spine. The incision is deepened through the subcutaneous tissues until the latissimus dorsi is met. This muscle is divided with coagulating diathermy taking care over haemostasis. The line of division is the same as for the skin. A plane of dissection is developed by hand under the scapula and serratus anterior. The ribs can be counted down from the highest palpable rib (which is usually the second) and the appropriate sixth rib periosteum is scored with the diathermy near its upper border. A periosteal elevator is used to lift the periosteum off the superior border of the rib. This reveals the pleura which may be entered by blunt dissection. A rib spreader is inserted between the ribs and opened gently to prevent fracture. Exposure may be facilitated by dividing the rib at the costal angle or by dividing the costotransverse ligament. Routine resection of a rib is an uncommon practice. The anaesthetist is now able to deflate the affected lung to allow a better view of the intrathoracic structures. In an emergency thoracotomy for penetrating wounds of the heart, a more anterior approach is used and no specialised supporting equipment is required (Fig. 9).
Large calibre (28-32 Fr) intercostal drains are usually inserted at the end of the procedure. It is common practice to site them through the seventh or eighth intercostal space anterior to the midaxillary line so that the patient does not lie on them.
For chronic management, such as closed drainage of empyema, the drains are tunnelled to come out more anteriorly for easier management. Traditionally, the more anteriorly sited drain goes to the apex and the posteriorly placed drain goes to the lung base. A rib approximator is used to realign the ribs and the stripped periosteum and intercostal muscle is sutured to the intercostal muscle below the stripped rib using a continuous absorbable suture. A nonabsorbable suture may be used to maintain the closure if healing is likely to be compromised. The fascia and muscle layer are closed in layers using an absorbable suture (Fig. 10). Skin closure is a matter of personal preference.
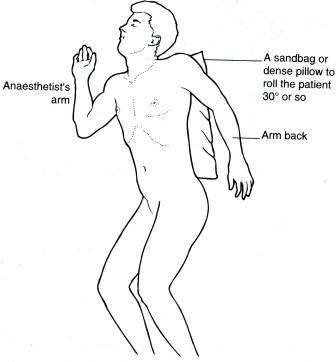
Fig. 9. Emergency left anterior thoracotomy for access to the heart. Requires no special supports or devices.
Analgesia is an important aspect of postoperative care and the process may be started intraoperatively by infiltrating the intercostal nerves in the region of the incision with a long-acting local anaesthetic. Various strategies have been developed to deliver analgesia postoperatively to facilitate a normal breathing pattern.
P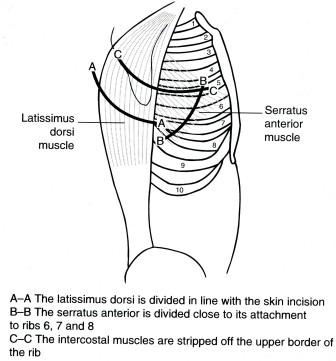 ostoperative
care. Lung
function is rarely assessed before urgent thoracotomy for trauma but
it is a vital part of the patient's preoperative work-up before
elective thoracotomy. A description of lung function testing is given
in Appendix 2 at the end of this chapter. These patients have limited
respiratory reserve following lung resection, so infection and fluid
overload are to be avoided. Chest drains placed at the time of
surgery drain blood collections and cope with air leaks if present.
Once the air leaks have settled and any remaining lung is
re-expanded, the drains are removed. Mobilisation, breathing
exercises and regular physiotherapy are begun as soon as the
patient's condition permits.
ostoperative
care. Lung
function is rarely assessed before urgent thoracotomy for trauma but
it is a vital part of the patient's preoperative work-up before
elective thoracotomy. A description of lung function testing is given
in Appendix 2 at the end of this chapter. These patients have limited
respiratory reserve following lung resection, so infection and fluid
overload are to be avoided. Chest drains placed at the time of
surgery drain blood collections and cope with air leaks if present.
Once the air leaks have settled and any remaining lung is
re-expanded, the drains are removed. Mobilisation, breathing
exercises and regular physiotherapy are begun as soon as the
patient's condition permits.
Fig. 10. Diagram of incision and layers encountered during antero-lateral thoracotomy.
Postoperative pain. It is important to deal with post-thoracotomy pain effectively so that a normal breathing pattern and gas exchange are to be achieved in the early postoperative period. Patient-controlled analgesia is an important development but still requires regular nursing and anaesthetic supervision. Internally placed catheters delivering local anaesthetic into the wound and beneath the pleura may also be effective. Long-term post-thoracotomy pain can be avoided by careful attention to detail during the operation. Sources of avoidable chronic pain include rib fracture and entrapment of intercostal nerves during wound closure.
Trauma accounts for most of the emergency workload of the thoracic surgeon but there are other situations encountered that demand prompt investigation and treatment.
Airway obstruction
Tracheal obstruction may present acutely as a life-threatening emergency or insidiously with little in the way of symptoms until critical narrowing and stridor occur. The more common causes of airway narrowing are outlined in Table 1.
Treatment depends on the underlying cause. Tracheal resection is a very specialised problem but in expert hands up to 6 cm of trachea may be resected without undue tension on the anastomosis. Tracheostomy may
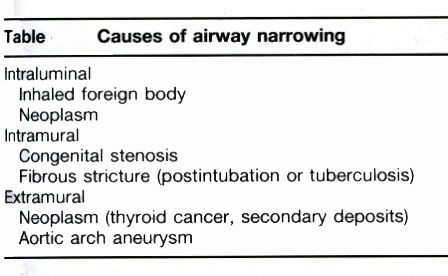
Table 1. Causes of airway narrowing
be required to overcome the obstruction but there are few indications to do this as an emergency and, in this situation, a cricothyroidotomy is probably preferable. Tracheal replacement with artificial substitutes has so far been unsuccessful. Sleeve resections of the major bronchi are also possible. Reversible airway obstruction (e.g. asthma) is the domain of respiratory physicians.
Inhaled foreign bodies
This is a regrettably common occurrence in small children and is often marked by a choking incident which then apparently passes. Surprisingly large objects can be inhaled and become lodged in the wider calibre and more vertically placed right main bronchus. If not removed, an obstructive emphysema may result but, if there is total occlusion of the bronchus, the air distally will be absorbed and the secretions may become infected. There are three possible presentations.
1. Asymptomatic
2. Wheezing (from airway narrowing) with a persistent cough and signs of obstructive emphysema
3. Pyrexia with a productive cough from pulmonary suppuration.
A chest radiograph is vital as the object may be radio-opaque. Often it is not radio-opaque or is obscured by the cardiac shadow or the inflammatory response. Bronchoscopy is required by an experienced operator with an experienced anaesthetist to administer the anaesthetic. The procedure may be very difficult if there is a severe inflammatory reaction. The rigid bronchoscope is best for retrieving inhaled foreign bodies. Failure to remove the object may necessitate a bronchotomy through a formal thoraco-tomy. If the object has caused chronic lung damage it may be necessary to remove the affected lobe.
Haemoptysis
There are a variety of conditions giving rise to repeated haemoptysis, including carcinoma, bronchiectasis, carcinoid tumours and certain infections. Severe rheumatic mitral stenosis is a rare cause. All patients with haemoptysis should be investigated at the very least by chest radiography and bronchoscopy and those with normal findings carefully followed up. Haemoptysis following trauma may be from a lung contusion or injury to a major airway. Severe haemoptysis is unusual and the treatment depends on the underlying cause.
ГОУ ВПО «Смоленская государственная медицинская академия
Федерального агентства по здравоохранению и социальному развитию»
МЕТОДИЧЕСКИЕ УКАЗАНИЯ ДЛЯ СТУДЕНТОВ
ПО ДИСЦИПЛИНЕ хирургические болезни
ACUTE ARTERIAL THROMBOEMBOLISM (thrombosis and embolism). VENOUS THROMBOSIS. PULMONARY EMBOLISM IN SURGICAL PATIENTS AND ITS PREVENTION
Составитель ассистент А.А.Безалтынных
Методические указания утверждены на методическом совещании кафедры госпитальной хирургии (протокол № 2 от 6 октября 2008 г.)
Зав. кафедрой______________(проф. С.А.Касумьян)
г.
THEME: ACUTE ARTERIAL OCCLUSION, VENOUS DISORDES
QUESTIONS FOR HOMEWORK:
1. Anatomy
2. Classification
3. Pathophysiology
4. Clinical symptoms.
5. Diagnostics.
6. Differential diagnosis.
7. Treatment.
8. Postoperative complications.
THE FUTURE
Angioscopy is becoming an important part of the vascular surgeons' armamentarium for both the diagnosis and treatment of peripheral vascular disease. By allowing the immediate detection and correction of technical errors and intravascular pathology its use may improve graft patency. For specific indications, angioscopy has also been shown to be an effective alternative to intraoperative angiography, but it is unlikely that angioscopy will replace angiography. As technological advancements improve angioscope systems and their use and indications widen, vascular surgeons will need to learn how best to use and interpret their findings. By carrying out routine angioscopy during arterial surgery, the vascular surgeon will be able rapidly to assess endovascular anatomy and thus potentially reduce graft failure rates and improve limb salvage.
Since heparin anticoagulation was found to have a beneficial effect in the treatment of thromboembolic disease there have been further efforts to improve the treatment of thrombotic disorders. Instead of using inhibitors of coagulation to shift the equilibrium of the clotting cascade, retarding the formation of thrombus with resulting slow absorption of clot, attempts were made to activate safely the endogenous serum fibrinolytic system. This goal can be achieved by the activation of the serum proteolytic precursor, plasminogen, to form plasmin. In the presence of fibrin substrate, plasmin fragments fibrin and promotes active thrombus dissolution.
THROMBOLYTIC AGENTS
Streptokinase
Streptokinase is a non-enzymatic polypeptide isolated from β-haemolytic streptococci and is the least expensive of the thrombolytic drugs. To initiate thrombolysis it must first combine with plasminogen in equal proportions to form an activated streptokinase;plasminogen complex that activates another plasminogen molecule to form plasmin. Sensitized individuals bear anti-streptococcal antibodies which inactivate streptokinase; the drug therefore has to be administered following a 100000 to 250000 IU loading dose. Intravenous doses of streptokinase average 100000 IU/hour, while local intra-arterial administration usually requires 5000 to 10000 IU/hour. The half-life is short (23 min); however, the effects of plasmin activation may persist for longer.
Urokinase
Urokinase is an enzymatic two-chain polypeptide produced by endothelial and renal tubular cells. Originally isolated from urine, where it is found in a concentration of approximately 6 IU/ml, it is now obtained from tissue cultures of human kidney cells. Since urokinase is an endogenous human protein and is not antigenic, in contrast to streptokinase it does not cause allergic side-effects such as pyrexia, anaphylaxis, rash, and serum sickness. Since urokinase does not complex with antibodies or plasminogen present in the serum, its dose effects are more predictable than those of streptokinase. Intravenous administration is commonly started by a bolus of 4400 IU/kg followed by infusion of 4400 IU/kg.h. Local intra-arterial doses vary between 1000 and 6000 IU/min. Like streptokinase, the half-life of the drug is short (16 min).
Tissue plasminogen activator and pro-urokinase
To minimize the bleeding complications associated with the use of thrombolytic drugs, efforts have been undertaken to find plasminogen activators which are much more efficient in the presence of fibrin. This would mean that the activity of the thrombolytic drug could be limited to the thrombotic process, and little systemic fibrinolysis would occur. The benefits of the drug's clinical activity could thus be optimized, while haemorrhagic complications could be reduced.
Tissue plasminogen activator was the first and thrombolytic agent to be used clinically and is produced using recombinant genetic technology. This glycosylated protease is a poor activator of plasminogen to plasmin, except in the presence of fibrin, when this reaction is greatly enhanced, apparently due to specific molecular conformational changes noted in vitro. This results in the production of high concentrations of plasmin in thrombus. Despite high hopes of tissue plasminogen activator being a very specific drug for thrombus dissolution, initial clinical trials have been disappointing. In addition, plasma half-life of the drug is very short (approximately 5 min).
Pro-urokinase is an inactive precursor of urokinase which is converted to urokinase by plasmin. This activation is slow except in the presence of fibrin or its split products; it is therefore thought to have thrombus selectivity and, because of its rapid inactivation by thrombin, its use should theoretically be associated with fewer haemorrhagic complications. The half-life of pro-urokinase is 3 to 6 min, but clinical experience with it is limited.
CLINICAL USE
Indications
Thrombolytic therapy is indicated for the treatment of venous thrombosis, peripheral arterial occlusion, and myocardial infarction. The clinical aim of thrombolytic therapy is to achieve a faster and more thorough dissolution of thrombus than can be achieved by anticoagulation alone. Most clinicians agree that anticoagulation is primarily of use in preventing thrombus propagation, not in thrombolysis.
Several important factors need to be considered in patients prior to thrombolytic therapy. First, there must be unequivocal evidence that a thrombus is causing the clinically important vascular occlusion. Clot lysis is most successful when the thrombus is fresh or only a few days old. When occlusions are arterial, the ischaemia must not be severe enough to require emergency surgical intervention. There must be no contraindications to the use of the thrombolytic drug: even with judicious use of these agents complications occur and these risks need to be weighed against the potential benefits of thrombolytic therapy.
Cardiac indications
Thrombolytic drugs may be used to relieve acute coronary thrombosis during myocardial infarction. The clinical goal is for immediate clot lysis with reperfusion of the ischaemic cardiac muscle to limit the extent of infarction.
The optimal method by which this goal can be achieved is by the intravenous administration of thrombolytic drugs shortly after the onset of cardiac symptoms. Minimizing the delay between onset of symptoms and administration of the drug increases the likelihood of limiting the infarct: the thrombolytic drugs that hold the greatest appeal for this application are those with clot fibrin. Tissue plasminogen activator has found its widest application in patients with acute myocardial infarction: it should be given within 4 h of onset of coronary symptoms, as a loading dose (10 mg intravenously), followed by 50 mg/h for the next 2 h. Streptokinase has been shown to be as effective as tissue plasminogen activator in recent trials.
Thrombolytic drugs have also been administered very successfully via the intracoronary route at the time of acute cardiac catheterization. In the 70 to 80 per cent of patients in whom coronary flow is successfully re-established anticoagulation diminishes the risk of reocclusion while the stenotic lesion that precipitated the original thrombosis is treated.
Venous indications
Venous thrombosis or thromboembolism is a common and potentially life threatening problem. In cases of deep venous thrombosis, the objectives of therapy are inhibition of clot propagation and embolism and minimizing the venous injury precipitated by the thrombus, thus reducing the morbidity associated with venous insufficiency and the postphlebitic syndrome. The standard therapy of anticoagulants, bedrest, and elevation of the extremities effectively achieves the former objectives. It does not, however, minimize the venous inflammation, scarring, and valve injury with subsequent venous hypertension.
The effectiveness of thrombolytic drugs and anticoagulation in patients with deep venous thrombosis of the lower extremity has been compared in several studies. The pooled results indicate that thrombolytic therapy lyses 47 per cent of clots compared with 6 per cent lysis achieved with anticoagulation alone. The mode of acute treatment did not affect the incidence of pulmonary emboli. The incidence of bleeding complications was four times higher in patients treated with thrombolytic therapy than in those treated with anticoagulation alone. Although it is clear that thrombolytic drugs are useful and effective for the acute removal of thrombus from veins, no published series has demonstrated a clinical advantage of this therapy to avoid the long-term complications of postphlebitic syndrome years after the illness. There is no long-term benefit in reducing venous valve incompetence with thrombolytic drugs in the treatment of deep venous thrombosis of the lower extremities. This makes justification for the use of thrombolytic drugs for this indication difficult, and we rarely use it in our practice.
The use of thrombolytic therapy in patients with a pulmonary embolus is controversial. The mainstay of therapy for acute minor pulmonary emboli remains heparin anticoagulation followed by several months of oral anticoagulation, and treatment of the precipitating cause of the venous thrombosis. In patients with acute massive pulmonary emboli, with significant haemodynamic compromise and pulmonary hypertension, thrombolytic therapy can cause a more rapid improvement in haemodynamics and in the angiographic appearance of the emboli. There is no evidence that its use improves mortality rates, and at 7 days there were no differences in lung scans of patients treated with heparin alone and those treated with thrombolytic drugs. Again, bleeding complications are more common in patients treated with thrombolytic drugs. In our practice we use thrombolytic therapy in those few patients without contraindications who have symptomatic pulmonary emboli with dyspnoea and hypoxia, but not in patients with severe right heart failure, who should have surgical embolectomy. Most patients are therefore treated with heparin, followed by coumadin anticoagulation as clinically indicated.
Subclavian or axillary vein thrombosis is a specific type of venous thrombosis that can be successfully treated with thrombolytic therapy; its use in this setting is less controversial. These thromboses can be precipitated by local intimal injury or effort thrombosis, thoracic outlet obstruction with venous compression, and by chronic indwelling venous catheters. Thrombolytic therapy has been used more frequently and with a higher degree of success in proximal upper extremity thrombosis for several reasons. Firstly, the clot is usually localized to the axillary vein;subclavian distribution, without distal extension down the arm veins. There is not usually a large number of venous tributaries present, in contrast to the situation when thrombus is present in the lower extremities. Another important factor is that catheters can readily be inserted into the axillary vein;subclavian venous thrombus, allowing the use of high dose local infusions that yield a higher lysis rate with a lower incidence of complications. Once recanalization of the vein has been established, the precipitating factor for the venous thrombosis can be ascertained and appropriately treated.
Arterial indications
The use of thrombolytic therapy in patients with an acute peripheral arterial thrombosis or embolus is controversial. It can, however, be very useful in the properly selected patient with an acute presentation, in whom ischaemic symptoms are not severe, where thrombus diffusely affects run-off, and when the risks for surgical intervention are high.
Before describing the criteria by which patients should be selected for arterial thrombolytic therapy, it is important to discuss its mode of administration. Historically, the drug was given intravenously to achieve a systemic thrombolytic state. Because the initial results were promising only for localized and acute artery occlusions, and were uniformly poor for thrombosed grafts, regimens of administration were altered to permit recovery of endogenous plasma levels of plasminogen. Therapy, which called for a high dose intravenous administration of the thrombolytic drug for several hours followed by a recovery time of 12 to 24 h and a repeat of the cycle several times.
These intravenous methods have been largely replaced by intra-arterial infusion of the thrombolytic drug directly into the thrombus. Local injection of the drug into the thrombus can activate intra-clot, or local plasminogen, increasing the rapidity of lysis without totally depleting systemic concentrations of plasminogen. Lower doses of thrombolytic agents can also be used with this route of administration. Technical success can also be predicted during such local administration by the ability to pass a guidewire through the occlusion in question. After thrombolysis, sheaths are already in place for balloon angioplasty, if the lesion is suitable for such treatment.
Although the technique by which thrombolytic therapy is administered is important, the aspect crucial to success is proper selection of patients. As well as recognizing an appropriate indication, it is important to determine that there are no contraindications to lytic therapy. Only patients who would otherwise be candidates for surgery because of their clinical presentations should be considered. This includes patients with an occluding thrombus precipitating a significant clinical end-organ ischaemia and a clinical presentation that would have a high likelihood of benefiting from its use.
Recent arterial occlusions are clearly more responsive to thrombolytic therapy than well-organized, older clot. Therefore, a new thrombus that forms during an interventional technique or manipulation is the most likely to lyse. Acute occlusions of bypass grafts can be recanalized with thrombolytic therapy, followed by identification and treatment of the precipitating cause of the thrombosis: preoperative identification of the precipitating factor facilitates the subsequent procedure. Long-standing prosthetic bypass occlusions can be recanalized since they do not scar down as do thrombosed vein grafts but retain organized thrombus at either anastomosis with resorbed thrombus in their midportion. Thrombolytic therapy is often useful in acute arterial or graft occlusion in the presence of loss of outflow vessels. Surgical thrombectomy of small or partially diseased arteries that have occluded as part of a graft or arterial thrombosis is often unsuccessful, and thrombolytic therapy can re-establish outflow before correction of the precipitating problem is attempted. In deciding whether to use thrombolytic therapy, other considerations include general surgical risk, complex anatomy, and scarring secondary to multiple operations.
Besides the usual contraindications to thrombolytic therapy there are specific contraindications for patients with arterial occlusions. Since implementation of the procedure and infusion to achieve clinical success can take up to 48 h, patients in whom end-organ viability is seriously threatened should not undergo thrombolytic therapy, but should immediately be taken to the operating room. Patients with early postoperative occlusion of a bypass graft should not be treated with thrombolytic therapy because of the high likelihood of substantial bleeding from the surgical site. An embolus in a surgically accessible vessel is not a good indication, especially when the added potential risk of further fragmenting an intracardiac thrombus and precipitating further emboli is considered. The presence of knitted Dacron grafts is considered a relative contraindication because of the reported incidence of bleeding through the graft wall if thrombolysis is performed before the graft is incorporated by the surrounding tissue (2-4 weeks).
Results of thrombolytic therapy for arterial or bypass occlusion varies with the technique, drug, and route of administration, but probably depend most of all on selection of patients. Experience from several units suggested that intravenous administration of thrombolytic therapy resulted in successful lysis in 38 per cent of 340 patients, with an incidence of major complications of 8 per cent. Intra-arterial administration of thrombolytic drugs does not change the complication rate (13 per cent), but more than doubles the success rate, to 78 per cent in 478 patients reviewed. These studies and their methods differed significantly and they include many early studies, performed when the technique was still evolving.
We exclusively use intra-arterial thrombolytic drugs for recanalizing occluded peripheral arteries or bypass grafts. Attempts at recanalizing native arterial occlusions are undertaken in patients with recent onset of symptoms and minimal atherosclerotic disease in the adjacent vessels, suggesting focal disease. Attempts at intra-arterial thrombolysis are more frequently performed in patients with recent occlusions of infrainguinal bypass grafts. These distal reconstructions are more likely to benefit from thrombolysis which allows the precipitating cause of the thrombosis to be determined, distal run-off that may have been lost at the time of occlusion to be re-established, and balloon angioplasty for correction of the underlying problem to be used. Suprainguinal bypass grafts, such as thrombosed limbs of aortofemoral grafts, present a different problem. Invariably, the occlusion results from a problem with the distal anastomosis, revision of which can be performed at the time of surgical thrombectomy of the limb of the aortofemoral graft. Thrombolysis is therefore of little benefit in this setting.
Intraoperative indications
Intraoperative thrombolytic therapy has been used when residual thrombus has been identified on intraoperative arteriography or when there is evidence of distal run-off loss. The reported series are small and hard to interpret since they are anecdotal, with no control group. Success of thrombolytic therapy is also variably defined, from angiographic criteria to restoration of pedal pulses. Arterial flow in these patients was invariably restored after administration of the drug. Our indications for thrombolytic therapy are the angiographic presence of retained thrombus after thrombectomy or known loss of distal run-off. Reported success averages 69 per cent, with a 14 per cent incidence of bleeding complications, and an amputation rate of approximately 15 per cent. Both streptokinase and urokinase are effective, but we have used a regimen of two slow injections of 100000 U of urokinase into the clamped distal circulation separated by 10 to 15 min.
MONITORING THROMBOLYTIC THERAPY
Before thrombolytic therapy is instituted, a standard haematological screen, including a complete blood count, prothrombin time, partial thromboplastin time, platelet count, thrombin time, and fibrinogen level should be performed to exclude an underlying coagulopathy. Once thrombolytic therapy is begun, haematological monitoring has two purposes: to ensure a lytic state, and to minimize haemorrhagic complications. To ensure a lytic state is necessary only if the procedure is unsuccessful. It is especially important if streptokinase is used, since high levels of antibodies can inactivate the drug and make the therapy ineffective. There are no good laboratory tests which can predict lysis or haemorrhagic problems. The thrombin time, which measures the clotting time of plasma after the administration of thrombin, is an indirect measure of the fibrinogen and fibrin split products: these can now be measured directly.
Some evidence suggests that low fibrinogen levels are associated with a higher incidence of haemorrhagic complications and that the patients who have serious problems are those with fibrinogen levels below 0.05 g/dl. It is therefore recommended that fibrinogen levels are checked and maintained above 0.05 g/dl, either by slowing the infusion of thrombolytic agent or by the administration of cryoprecipitate. Despite these published data, we have not used clinical laboratory testing during thrombolytic therapy other than a routine haematological screen to exclude a coagulopathy. The results of these tests are difficult to obtain rapidly, and careful clinical evaluation in an ICU setting for evidence of bleeding has resulted in minimal complications.
Complications of thrombolytic therapy
Some complications of thrombolytic therapy are common to all of the drugs, while others are specific to an individual drug. Immunological reactions such as pyrexia, anaphylaxis, serum sickness, or a rash are limited to streptokinase because of its antigenic source: such complications are treated by stopping the infusion, with supportive care to treat the symptoms.
The most common complication is haemorrhage. Because bleeding is unpredictable, its potential occurrence is the major criterion for exclusion of patients from thrombolytic therapy. Bleeding can occur at the site of drug administration, from other puncture sites, or from areas of recent surgery as well as spontaneously in other anatomical areas. Treatment consists of stopping the infusion of the thrombolytic drug, treating the haemorrhagic area as warranted, and, if necessary, transfusing blood products to replenish coagulation factors. If bleeding is serious, cryoprecipitate, the only source of fibrinogen, must be replenished along with fresh frozen plasma. Administration of Σ-aminocaprionic acid is not usually needed since the half-life of the thrombolytic agents is very short.
The administration of heparin during thrombolytic therapy to inhibit the formation of thrombus around catheters, or keep the thrombus from propagating has been said to increase the incidence of haemorrhagic complications. However, with the use of intra-arterial catheters, heparin decreases catheter thrombosis and does not appear to increase the incidence of haemorrhagic complications.
Embolism is a potentially serious complication, which has been inconsistently reported, but may have an incidence as high as 50 per cent. It can occur as a result of thrombus formation around the catheters used in intra-arterial infusion, the partial dissolution of thrombus and distal embolization in the same vessel, or as a wash-over from the thrombus into a more proximal vessel secondary to the breakup of clot and infusion into an occluded artery. These emboli may be clinically silent; however, they can temporarily produce worsening of ischaemia during the procedure. If emboli are identified, they are treated with continued thrombolytic therapy to the embolus, but if there is no clinical improvement surgical intervention may be required.
FUTURE TRENDS OF THROMBOLYTIC THERAPY
As thrombolytic therapy has been used for over two decades, advances continue. New drugs may be more selective in activating clot specific plasminogen without causing a systemic activation of the thrombolytic system. New and better delivery methods are being developed: coaxial systems that inject the drug at high speeds through many small side holes improve delivery of the drug and diminish the time of administration. New mechanical devices are being developed that work along with the thrombolytic drugs to break up the clot and remove it by suction. There are also pulsed laser systems that can selectively ablate thrombus, thereby enhancing the efficiency of the thrombolytic drug. Continued research will make thrombolytic therapy more widespread and safer in its applications for the treatment of thrombotic disorders.
DEFFINITION AND AETIOLOGY
An embolus consists of undissolved material which is carried in the circulation and impacts in a blood vessel, usually blocking it. The most common source of arterial embolism is the left atrium in atrial fibrillation, accounting for two-thirds of all cases. Thrombus forms because of stasis in the enlarged and fibrillating atrium, and fragments detach to enter the arterial circulation. Thrombi can also form on the damaged endocardium of the left ventricle after myocardial infarction, and arrhythmias cause these to detach and embolize. Emboli can arise from the aorta and its branches. Atheromatous plaques may rupture, allowing cholesterol debris to pass distally and block small arteries. Platelet thrombi forming on ulcerated atheromatous stenoses may also embolize. Thrombus forming in aneurysms due to abnormal patterns of blood flow can also form an embolism and this is a particular danger in the case of popliteal aneurysms.
SITES AFFECTED BY EMBOLI
Emboli usually lodge at the bifurcations of arteries, because the diameter of each major branch is less than that of the main branching vessel.
Emboli to the limbs
In surgical practice most arterial emboli affect the limbs, the leg being affected six times more often than the arm. Limb emboli are considered in detail below.
The brain and the eye
The brain is a frequent site of embolism, resulting in stroke. Small emboli passing up the internal carotid artery may also enter the retinal vessels, causing temporary or permanent blindness. If carotid atheroma is the source for recurrent emboli to the eye or brain then carotid endarterectomy may be required, although many patients are treated by antithrombotic drugs such as aspirin.
Mesenteric emboli
The superior mesenteric artery is usually affected, with clinical features of severe abdominal pain, sometimes vomiting or diarrhoea, and evidence of an embolic source. Other features are a high white blood cell count and elevated serum amylase. A plain abdominal radiograph will show the absence of normal small bowel gas shadows centrally.
Urgent laparotomy is required and, if the bowel is still viable, embolectomy of the superior mesenteric artery can be performed. If there is doubt about bowel viability a laparotomy should be performed 24 h later. In many cases the bowel is not viable and extensive resection is required. These patients require careful attention to replacement of fluid, electrolytes, and blood. If the whole small bowel has infarcted the patient will die.
PATHOPHYSIOLOGY
The effects of an embolus blocking a major limb artery depend on the level of the obstruction and on the capacity of collateral arteries to carry blood to the distal tissues. In the absence of good collateral flow there is stasis of blood in the arteries beyond the block and propagated clotting occurs. Propagated clot also extends proximally to the next major branch. Reflex spasm of distal arteries is another effect of acute arterial occlusion. Clotting and spasm both make ischaemia worse.
Acute ischaemia due to an embolus causes hypoxia of the tissues and a failure to remove waste products; these are particularly damaging to muscle cells which have a rapid metabolic rate. Muscle death starts to occur after about 6 h. Initially ischaemia causes pain due to accumulation of metabolites, but as peripheral nerves become increasingly hypoxic paraesthesiae and eventually complete anaesthesia of the extremity occurs.
If the occlusion remains unresolved, venous thrombosis results from stagnation of blood flow: this is a late feature associated with a poor prognosis.Fixed staining of the skin due to extravasation of blood into the tissues is another late sign. Continued neglect results in gangrene.
CLINICAL ASSESSMENT
Diagnosis of embolism causing acute ischaemia of a limb. The clinical features are best remembered as the pain, pallor, pulselessness, paraesthesiae, paralysis, and perishing cold. The onset of symptoms caused by an embolus is sudden, with pain and pallor occurring first. Colour change is variable and depends on the amount of collateral blood flow. If there are no established collaterals the extremity is white, sometimes with a bluish tinge. If some blood flow is maintained a pink colour remains, but capillary return is slower than normal. The more profound the ischaemia, the sooner paraesthesiae will be followed by anaesthesia. Loss of sensation is a serious sign and an indication for urgent treatment to restore blood flow. Paralysis is also a sign of advanced ischaemia.
Pulses distal to the occlusion are lost, while immediately proximal to the occlusion the pulse may be enhanced due to the high resistance caused by obstruction.
Acute ischaemia due to an embolus is a clinical diagnosis and special tests should not be necessary, but Doppler ultrasound investigation confirms absent or poor blood flow signals in the distal arteries, and the systolic pressure is unrecordable or low. An arteriogram is not necessary in the presence of an obvious embolic source (for example atrial fibrillation) and clear evidence of a sudden arterial occlusion, but angiography is worthwhile if there is any doubt about the diagnosis.
Differential diagnosis
The main differential diagnosis is acute thrombosis occurring in arteries already narrowed by atherosclerosis (often called thrombosis in situ or acute-on-chronic ischaemia). The onset of ischaemia is often less sudden than in embolism and the degree of ischaemia is less profound, with preservation of a pink colour to the skin and intact sensation, due to established collateral arteries. There may be evidence of chronic arterial disease with a history of intermittent claudication, and absence of pulses with reduced systolic pressures in the contralateral limb. The absence of an obvious embolic source also supports a diagnosis of thrombosis rather than embolism. A generalized illness with hypotension or dehydration may be evident as a precipitating cause for acute thrombosis.
The distinction between embolism and thrombosis is not always easy, and in any doubtful case an arteriogram should be performed. If arteriography suggests thrombosis, then this can be treated by low dose infusion of a thrombolytic agent (for example streptokinase) through an arterial catheter. This method of treatment requires radiological facilities and expertise, and haematological monitoring of blood clotting during the infusion of the thrombolytic agent. When the thrombus has been lysed, transluminal angioplasty or bypass grafting may be indicated for repair of the underlying arterial stenoses.
Deep vein thrombosis is sometimes confused with arterial embolism, because it causes pain, reduced ability to move the limb, and colour change. This differential diagnosis should not be difficult: the limb with a venous thrombosis is swollen, sensation is not lost, and Doppler examination will confirm a normal arterial supply. Venous thrombosis occurring as a late sequel of arterial occlusion is accompanied by florid signs of neglected ischaemia.
TREATMENT
The first priority is relief of pain using strong analgesics such as morphine or pethidine (meperidine) given intramuscularly. An intravenous bolus of heparin should be given to reduce propagated clotting (5000 units;10000 units). Continuous infusion of intravenous heparin is then commenced if any delay is anticipated in definitive treatment.
The ischaemic limb should never be actively warmed this accelerates tissue damage. Measures to cool the limb are sometimes recommended but are generally impracticable. Another traditional recommendation is to nurse the patient with the limb dependent but any improvement from such a manoeuvre is likely to be marginal. Treatment for any acute medical condition, such as cardiac failure or arrhythmias, is commenced.
After these initial steps the aim should be emergency embolectomy as soon as possible to re-establish blood flow to the extremity. Embolectomy is usually performed under a local anaesthetic because most patients are elderly and unfit. The area to be anaesthetized should be marked and a dilute local anaesthetic, for example 0.5 per cent lignocaine (lidocaine), chosen so that a large volume can be used. It is helpful, but not essential, to have an anaesthetist in attendance to monitor the patient (ECG and blood gases) and to administer oxygen or sedation if this is required. An intravenous infusion should be set up before starting the operation.
Transfemoral embolectomy is the best initial approach for all lower limb emboli, while in the upper limb exposure of the brachial artery is required. For femoral embolectomy the incision should be vertical over the femoral artery, and should allow easy access up to the inguinal ligament. The common femoral artery is controlled with a sling as high as possible. Its bifurcation is exposed and slings are passed around the superficial and profunda femoris arteries, and also around any other branches.
In the upper limb it is easiest to approach the brachial artery through a longitudinal incision over the medial aspect of the upper arm, and this usually gives a satisfactory result. An approach through the antecubital fossa allows separate embolectomy of radial and ulnar arteries. This involves an S-shaped incision (medial above the elbow, curving across the antecubital fossa to the lateral aspect of the forearm). The bicipital aponeurosis is divided to reach the brachial bifurcation.
If the artery is non-pulsatile it is opened without any clamps in place. For simple embolectomy transverse incisions in arteries allow closure without the risk of narrowing the lumen. Longitudinal arteriotomy gives greater scope for reconstructive surgery but may require closure with a patch to avoid stenosis in narrow vessels.
A balloon embolectomy catheter of appropriate size is selected and the balloon is tested by inflating it with fluid (from a 1 ml or 2 ml syringe). The catheter is passed, proximally if it is necessary to remove clot and to ensure good inflow, and a proximal clamp is applied. After passing the catheter through an iliac clot into the aorta the contralateral limb must be checked at the end of the operation to ensure that embolic material has not been dislodged distally through the opposite iliac system.
Balloon catheters must be used gently, especially in vessels roughened by atheroma, to avoid intimal damage. The stilette should always be removed before passing the catheter. It should be possible to pass a catheter to the foot and passages are repeated until no further thrombus or clot is withdrawn. A balloon catheter should also be passed down the profunda femoris artery in the thigh. Distal flushing with about 100 ml heparinized saline (from 500 ml normal saline containing 5000 units heparin) is then performed through a soft catheter (for example a number 6 umbilical catheter) passed into the distal artery.
The patient should be warned to expect some discomfort during balloon withdrawal and also perhaps during flushing of the extremity with cold heparinized saline.
The arteriotomy is closed with a continuous non-absorbable suture (e.g. 0000 or 00000 polypropylene), checking inflow before final closure.
On releasing clamps the extremity should rapidly regain a pink colour with return of palpable pulses. If the result is unsatisfactory (the catheter fails to pass far enough or the foot is not improved) then an immediate arteriogram should be undertaken on the operating table with a proximal clamp in place to demonstrate the state of the distal vessels. The options thereafter are exposure of the popliteal artery for embolectomy or bypass surgery, or the use of thrombolytic agents. These are best performed by a surgeon with vascular expertise. If the expertise is not available and the foot is viable, then intravenous heparin therapy should be instituted.
If the material removed from the arteries is not typical thrombus it should be sent for histology to exclude a tumour embolus.
Swelling of the leg may occur after revascularization, and this can cause increased compartmental pressure leading to muscle necrosis if untreated. If there is any suspicion of raised intracompartmental pressure, a generous fasciotomy should be done. Compartment syndromes are more common after reconstruction for vascular trauma than after embolectomy.
Other methods of treating emboli
Embolectomy is the best treatment for arterial emboli. If in doubt about the management of an acutely ischaemic limb the artery should be explored with a view to an embolectomy.
Thrombolytic therapy
Low dose intra-arterial streptokinase or urokinase can be effective in the treatment of embolism as for arterial thrombosis. This technique should only be used if the extremity is viable (sensation preserved). The infusion should be stopped after 48 h if there is no improvement, but if sequential radiographs show lysis then the infusion may be continued for up to 5 days. This method requires good radiological support.
Heparin therapy
Intravenous heparin alone can be used for the treatment of patients with acute limb ischaemia provided the limb is viable. This is safe management, especially when the facilities or expertise for vascular work are limited, or while awaiting a vascular opinion. Heparin minimizes propagated clotting and improvement may occur through natural clot lysis and the development of collaterals. The eventual state of the circulation to the limb is likely to be less good following heparin therapy alone than after successful embolectomy.
Percutaneous aspiration thromboembolectomy
This technique employs a specially designed catheter sheath system which can be used alone or in combination with thrombolytic drugs. The catheter is used to aspirate fragments of thrombus from the distal arteries and it is best suited to treating iatrogenic emboli resulting from intra-arterial catheterization or balloon angioplasty. This method is currently confined to specialist units.
Long-term anticoagulation after embolism
Administration of intravenous heparin in the immediate postoperative period reduces early recurrence of emboli. Thereafter, long-term anticoagulation is traditionally instituted (warfarin by daily oral administration) in the hope of preventing further emboli. Oral anticoagulants certainly should be used in patients in atrial fibrillation, but for patients with no obvious embolic source there is conflicting evidence of effect. Nevertheless, anticoagulation seems reasonable for all patients who will take warfarin reliably and from whom regular blood samples can be obtained for clotting studies. If haematological monitoring is difficult or if patients are unlikely to comply properly then the risk of haemorrhagic complications probably outweighs the benefits of long-term anticoagulation.
LATE PRESENTATION OF ARTERIAL EMBOLI
Patients with emboli may present many days after the acute event. Embolectomy may be successful even after a delay of a month but is rarely successful thereafter, because thrombus adheres to the vessel wall. Thrombolytic therapy may also be successful up to 1 month after impaction of an embolus.
Thrombolysis or intravenous heparin therapy are often used for these patients who present late but whose limbs have remained viable. If the obstruction is not relieved then arterial reconstruction can be performed at a later date if required.
THE MORTALITY AND MORBIDITY OF PERIPHERAL EMBOLI
Patients suffering embolism are often elderly and infirm, and up to 30 per cent die in the postoperative period. Factors associated with a higher mortality are increasing age, recent myocardial infarction, proximal (aortoiliac) occlusions, poor cardiac and pulmonary function, and pre-existing arterial disease.
Amputation is an uncommon sequel to embolism and is associated with delayed presentation. Limb loss following acute ischaemia is more commonly associated with thrombosis in diseased arteries than with emboli.
MICROEMBOLI
The small emboli that arise from arteriosclerotic arteries or aneurysms present either as isolated ischaemic digits 350 or as ischaemic patches on an extremity. The main differential diagnoses are vasculitis and haematological disorders such as thrombocythaemia. Trash foot is an important complication of grafting for aortic aneurysm and describes the passage of a shower of loose thrombus into the small vessels of the feet.
The veins of the lower limb must not be viewed as a series of inert venous conduits but rather as a complex pumping mechanism capable of returning venous blood to the heart against the force of gravity in the upright position. If active thrombosis is excluded, it is some form of failure in this mechanism that underlies nearly all venous disorders in the lower limb. The essentials of normal anatomy and physiology will be described first, especially those aspects that may undergo change and cause venous problems.
The imaging techniques. Functional phlebography and ultrasonography by Duplex or colour flow scanning
If the special tests with instruments have not satisfactorily explained the nature of the venous disorder then a technique visualizing the veins can be used. This will not only display abnormal outline to the veins, such as occlusion or deformity, but will show if valves are defective or absent. In addition, abnormal patterns of superficial or deep venous flow, in response to exercise, can be demonstrated. The two main techniques are functional phlebography and ultrasonography.
Functional phlebography
Phlebography has been transformed over recent years by several factors, particularly the use of a non-irritating osmolar opaque medium, such as Iohexol, which causes no discomfort and does not precipitate phlebitis, the introduction of the image intensifier which allows prolonged viewing without exposing the patient to excessive radiation, and the realization that the patient must be examined in the position that causes venous problems, that is to say, upright. With the exception of acute thrombosis in the deep veins, it is only when the patient is near upright and exercising intermittently that many features of venous disorder become apparent. The essence of functional phlebography is to study the functioning of the veins whilst they are working to return blood against gravity. This will allow valve function to be gauged and the causes for inadequate musculovenous pumping to be identified; occlusion or severe deformity in deep veins can be recognized, unnatural enlargement or tortuosity can be seen, and unusual flow patterns, for example collateral flow in superficial veins, are recognized. It is essential that the pattern of venous return should not be distorted by using such artefacts as constricting bands. The opportunity to witness events is very brief and is limited by the fact that only part of the limb can be viewed at one moment, the need to keep the amount of opaque medium within safe limits, and the rapidity with which the changes come and go with exercise. It is not really possible for a radiologist to give comprehensive functional examination of a limb without knowing the likely nature of the problem that he should concentrate upon. It is essential that he should be well briefed by the surgeon or, even better, that the surgeon should be present, guiding the radiologist through the features to be looked for. For these reasons, functional phlebography is a skilled and rather specialized examination. It is important to record the rapidly changing events in some fashion for subsequent study and much can be learnt from this. Depending on the sophistication of apparatus, either multiple static films can be taken or the whole procedure recorded videographically. One problem is to know the relationship in depth of veins one to another and misinterpretation is all too easy without some method of clarification. The simplest way is to take two views of the limb in different rotations so that the relative shift in the veins gives an immediate understanding of their relationship. Before starting, various manoeuvres are rehearsed with the patient, such as exercise by rising up on the toes or rotating the limb inwards and outwards. Any movement by the patient causes immediate changes within the veins, dispersing the opacified blood and quickly creating a confused picture, and for this reason requests to exercise or rotate the limb must be carefully timed. Throughout the examination it must be remembered that only streams of opacified blood are seen and it is quite possible for a large vein to remain invisible because the opacified stream has chosen other channels; failure of a known vein to appear does not necessarily mean that it is occluded and different ways may have to be found to persuade opacified blood to enter it. Functional phlebography is not an easy method but it is immensely rewarding to those who familiarize themselves with it. It is not possible here to describe functional phlebography in detail, but some of the main manoeuvres will be outlined and examples of the results that can be obtained are given in the sections upon the various disorders. The method preferred by the Oxford Vascular Service is described here, but other centres have evolved their particular techniques for dynamic phlebography and obtain similar results.
Functional phlebography in the normal
If the patient is tilted foot down to 50° or more and contrast medium is introduced by needle into a superficial vein on the calf, it will be seen to drift down the superficial veins until checked by a valve and soon appear in the deep veins of the leg. This downward movement is because the specific gravity of the opaque medium is higher than that of blood. If injection is continued up to, say, 100 ml, the deep veins will fill steadily so that they are clearly outlined up to the iliac veins and beyond. Even a small exercise movement will cause rapid movement upwards in the deep veins with partial emptying of the superficial veins and segmentation of opacified blood between their valves. If movement continues the picture will quickly become confused and all opaque medium will soon leave the limb. During the phase of static filling, opacified blood enters tibial and peroneal veins by multiple perforators, but often it will similarly enter large venous sinuses, the pumping chambers, lying within muscles. This is a normal phenomenon but, for instance, if a single large gastrocnemius vein fills in this way it may be mistaken for a short saphenous vein. Static filling of the deep veins, as just described, is the first stage in functional phlebography and will test the ability of the deep veins to fill normally. Judicious exercise movements may be given during this stage and subsequently to study the patterns of flow. The valves of the deep veins can be shown by lowering the head of the table to near horizontal, so that the veins deflate slightly; the table is then rapidly returned to near vertical again (the swill test), which causes the valve cusps to fill and gives a clear impression of their number and, to some extent, their competence.
Varicography
In superficial incompetence this method is of value in showing the source of incompetence, for example, a short saphenous termination or a recurrent set of varicose veins of uncertain origin. This method gives little information on function but it can be combined with information gained from functional phlebography to give comprehensive views of the veins at fault. However, the filling of veins by varicography is capricious and results can be misleading until experience is gained.
Ultrasonography (ultrasonic imaging)
Pulsed beam ultrasound scanning creates an image of tissue interfaces and moving blood on a video display unit. This can be used to portray a section through arteries and veins in any plane, from horizontal to vertical. The plane is chosen to show the structure under study to best advantage. In this way a portion of vein over some inches may be shown with its walls and valves clearly outlined and any movements by them demonstrated. The direction and speed of blood flow within the vessel at any point can be individually picked out by a Doppler flow facility and recorded separately (duplex); colour flow scanning (triplex) has the additional feature of displaying flow in a colour representing its direction of movement and showing the velocity by the intensity of the colour. Thus, the vein walls and lumen can be outlined and flow studied, clot may be recognized by absence of flow and immobile vein walls, and the structure of valves and their competence can be scrutinized in detail. The anatomy of veins can be visualized preoperatively, for example, to demonstrate the level and manner of short saphenous termination; individual deep veins and their branches can be distinguished and flow patterns displayed, so that, for instance, incompetence in a gastrocnemius vein can be recognized (immediately apparent by colour flow) and the reflux within it estimated to assess its significance. This is possible because the diameter and speed of flow within any designated vessel can be measured and from this the volume of flow, either expelled upwards or refluxed downwards, can be calculated. In the popliteal vein this will indicate the effectiveness of the musculovenous pump below the knee or, conversely, the severity of reflux in the deep veins at that level. The advantages of ultrasonography are that it is non-invasive and can be used for prolonged viewing with repeated cycles of exercise in a way not possible with phlebography and, moreover, the running costs are decidedly less. It is an excellent method for special study of localized areas of vein, both for research and in the practical management of some venous problems. It is already a practical alternative to phlebology for detecting acute thrombosis or in the display of specific structures, such as a short saphenous termination or a valve suspected of leaking. Its potential for future development is considerable and eventually it may displace phlebology for many purposes in the lower limb.
Superficial thrombophlebitis (phlebothrombosis)
Thrombosis in varicose veins is quite common and may be accepted as a complication without any very serious implications. However, when thrombosis occurs spontaneously in previously normal veins, it may well signify a serious background condition hitherto unsuspected. This includes malignancy (for example, in the pancreas or bronchus), leukaemia or polycythaemia, vascular disease (especially thromboangiitis obliterans), and disorders of blood clotting. Whether it appears as single episode or recurring episodes in different parts of the body (phlebitis migrans), it is a clear indication for full medical screening to identify the cause. Varicose veins in the lower limb, however, can be regarded as sufficient explanation for local thrombosis without necessarily searching further for an underlying cause.
Thrombosis within a varicose vein, or any superficial vein, is accompanied by a tender swelling, often red and slightly warm to the touch, and until quite recently this was commonly regarded as an inflammation of the vein caused by infection. As explained above, the old term of phlebitis was retained until it was realized this was not so and that it must be distinguished from septic thrombosis in a vein secondary to a surrounding infection and which, by suppuration, is liable to cause a dangerous pyaemia. Now that it is recognized that the condition is a response to the thrombosis itself, the terms superficial thrombophlebitis or phlebothrombosis are used to denote this. Varicose veins are undoubtedly prone to thrombosis, partly because there are long periods of stasis in the unnaturally enlarged vein, for example when sitting, but also because production of fibrinolysin in the walls of a varicose vein is reduced. Even when quite a large thrombus has formed, blood continues to stream by it, continually depositing further thrombus so that the vein becomes progressively distended with clot. This does not, however, completely occlude the vein and studies by venography or by Doppler flowmeter show that, when the patient is upright and moving, blood continues to flow down the varicose vein, infiltrating its way round the clot and continually depositing further thrombus. The vein becomes painfully over-distended and shows an inflammatory reaction. This gives the key to treatment, which is to apply external compression to prevent blood from continuing to flow through the affected vein. The process may limit itself to a few centimetres of vein or may go on extending progressively to involve a considerable area, perhaps the entire calf, when it may be mistaken for a deep vein thrombosis. It may extend into the saphenous vein and along its full length, and in these circumstances, occasionally, may release a pulmonary embolus. Apart from this, it is seldom life-threatening although very troublesome to the patient.
Diagnosis
The tender red swelling on the leg, with an indurated cord of thrombosed vein is characteristic. The diagnosis can usually be safely made on clinical grounds alone but where deep vein thrombosis is seriously suspected, this should be checked by phlebography or ultrasonography rather than risk mistreating a life-threatening condition. Occasionally it is difficult to distinguish between superficial thrombosis in the long saphenous vein and a lymphangiitis but the latter condition will lack the firm cord of thrombosed vein and will be accompanied by high fever unlike the low pyrexia that may be present in superficial phlebitis.
Treatment
Traditional conservative treatment is by applying a firm compression bandage (as in sclerotherapy). An anti-inflammatory agent such as indomethacin may be used to relieve pain but antibiotics are not necessary. If the condition is unusually extensive it may be advisable to put the leg in high elevation (but check for ankle pulses beforehand), with the patient encouraged in active movements and maintained like this until the condition starts to recede; at this stage mobilization is commenced in a firm support bandage. In most circumstances anticoagulants need not be used but in extreme cases the condition will resolve more rapidly with a few days of heparin followed by oral anticoagulation for some weeks.
However, active intervention is often greatly to be preferred because it will bring immediate relief from pain and ensure a rapid recovery. If a substantial set of varicose veins are present and the circumstances allow it, the most effective course is to carry out the appropriate operation for this without delay and at the same time to excise the thrombophlebitic vein; this will be curative for the phlebitis and the varicose state that caused it. When this course is not possible, the simple procedure of evacuating the clot from the thrombosed vein is very effective and may be carried out under local anaesthesia by a single 2-mm skin incision into it. The clot is then extruded by firm finger pressure and a pad and bandage applied without skin suture. This gives complete relief and, provided firm compression is maintained for several weeks afterwards, will cure the phlebitis so that the overall problem of widespread varicose veins can be tackled at a more convenient time.
Treatment
Provided that phlebography has shown the deep veins to be widely open but valveless, it is permissible to remove enlarged and valveless superficial veins. In borderline cases, this reduction in the load on the pumping mechanism may restore it to an adequate performance. In more severe examples it brings little or no benefit and treatment will have to rely upon the conservative measures of elevation whenever possible and the use of external support by elastic stockings.
PATHOGENESIS OF THROMBOSIS AND THROMBOLYSIS
Thrombosis is usually initiated when alterations in the vascular endothelium cause platelets to adhere to subendothelial connective tissue. Collagen binds to platelet receptors to activate enzymes that catalyse the release of arachidonic acid. Cyclo-oxygenase converts arachidonic acid to thromboxane A, which increases phospholipase C activity and stimulates platelet activation and secretion. Cyclo-oxygenase is inhibited by aspirin and non-steroidal anti-inflammatory agents. Prostacyclin, in contrast to thromboxane A, is produced from arachidonic acid by endothelial cells and inhibits phospholipase C activity and platelet activation. Platelets release a variety of mediators after activation, including adenosine diphosphate which modifies the platelet surface, allowing fibrinogen to attach and link to adjacent platelets. Platelet-derived growth factor is also released, stimulating the growth and migration of fibroblasts and smooth muscle cells within the vessel wall.
The primary haemostatic plug is strengthened after several minutes by activation of the coagulation pathway which causes the production of thrombin and conversion of plasma fibrinogen to fibrin. The reactions require formation of a surface-bound complex and the activation of proteases by proteolysis: they are regulated by plasma and cellular cofactors and by calcium. For example, the local concentration of reactants is reduced by the flow of blood and increased by stasis. In addition, the absorption of coagulation factors to cellular surfaces and the presence of multiple inhibitors in plasma inhibit thrombosis.
Thrombolysis begins immediately after formation of the haemostatic plug. The principal physiological factor, tissue plasminogen activator, diffuses from endothelial cells to convert plasminogen absorbed to the thrombus into plasmin, which degrades the fibrin polymer. Plasmin can also degrade fibrinogen, but systemic fibrinolysis does not occur because tissue plasminogen activator activates plasminogen more effectively when it is absorbed to fibrin thrombi. Furthermore, excess plasmin is rapidly bound and neutralized by plasmin inhibitor, and endothelial cells also release an inhibitor of plasminogen activator.
A variety of factors may be responsible for the development of thrombosis in the absence of endothelial injury. Low blood flow increases local concentrations of coagulation reactants. This may result from venous varicosities, prolonged bed rest, or stasis during and after an operation or illness. Factors that diminish arterial blood flow include cardiac disease or shock. Congenital or acquired abnormalities in the fibrinolytic system and certain dysfibrinogenaemias also predispose to intravascular thrombosis. While the last two specific disorders are of extreme interest and help to elucidate the complex mechanisms of thrombosis formation, they are identified in only 10 per cent of patients with thrombosis.
Antithrombin forms complexes with all of the serine protease coagulation factors except factor VII. The anticoagulant effect of heparin is due to acceleration of the rate of formation of this complex. The most common antithrombin deficiency causes a mild defect in 1 of 2000 individuals. Patients with antithrombin deficiency can develop acute thrombosis or embolism which should be treated with intravenous heparin, since they usually have sufficient functional antithrombin to act as a heparin cofactor. Subsequent treatment with oral anticoagulants prevents recurrent thrombosis. Relatives of the patient should also be screened, and those found to have low antithrombin levels should receive proplylactic anticoagulation with heparin or plasma infusions prior to surgical procedures in order to raise the antithrombin III level and decrease the risk of thrombosis. Chronic oral anticoagulation is not recommended unless a clinical thrombotic episode occurs.
Protein C is converted to an active protease by thrombin after binding to thrombomodulin. Activated protein C inactivates plasma cofactors V and VIII and stimulates the release of tissue plasminogen activator from endothelial cells. The inhibitory function of protein C is enhanced by protein S. Patients with acute thrombosis and moderate protein C or S deficiency should receive heparin followed by oral anticoagulants, although treatment with warfarin may further reduce the concentration of both proteins C and S. Deficiency in proteins C or S may predispose to the rare but serious complication of warfarin-induced skin necrosis. Homozygosity for protein C deficiency is rare, but can be a cause of intravascular coagulation in the neonate. These patients may require periodic plasma infusions rather than oral anticoagulants to prevent recurrent intravascular coagulation and thrombosis. Defects in fibrinogen or plasminogen or decreased synthesis or release of tissue plasminogen activator have been described. Patients with these disorders, as well as those with abnormal plasminogen, have been successfully treated with heparin and oral anticoagulants.
In addition to the heritable disorders, many common conditions and illnesses are associated with an increased risk of thrombosis. Age is an important factor in the development of thrombosis, and pulmonary embolism primarily affects the middle-aged and elderly. Cardiac disorders, especially congestive heart failure, acute myocardial infarction, and atrial fibrillation, are particularly conducive to the development of pulmonary embolism. Metastatic malignancy, particularly carcinoma of the pancreas and prostate, is also associated with an increased incidence of pulmonary embolism. Patients undergoing major surgical procedures or suffering major trauma or burns also have an increased risk of venous thrombosis. Factors produced in damaged or ischaemic tissues or in patients with metastatic disease, together with venous stasis and endothelial injury, induce the formation of venous thrombi.
Pregnancy increases the risk of pulmonary embolism because pressure from the gravid uterus retards venous flow from the legs and pelvis. Postpartum infection may also predispose to septic thrombophlebitis and embolism, and oral contraceptives, which lower antithrombin III levels, are also associated with the occurrence of pulmonary embolism. Several haematological disorders which cause poorly defined abnormalities in circulating leucocytes and platelets predispose patients to venous thrombosis. Diseases which affect the endothelial cells, or the administration of drugs such as l-asparaginase, which inhibits production of multiple coagulation factors, may also predispose patients to thrombosis.
VENOUS THROMBOSIS
Haemostatic thrombi that form in veins when blood flow is reduced are richly endowed with fibrin and entrapped blood cells and contain relatively few platelets. These are often called red thrombi. The friable ends of these thrombi which form in leg veins often break off and cause emboli in the pulmonary circulation. Thrombus formation is typically asymptomatic and the site does not usually become inflamed. Inflammation of a thrombus in a vein is called thrombophlebitis. The acute inflammatory response makes the developing thrombus firmly adherent to the intima of the vessel wall, making embolization uncommon.
Venous thrombosis may involve the superficial or deep venous systems of the leg. When both systems are affected, thrombus formation usually begins in the deep veins and extends to the superficial system. Varicose veins are often associated with superficial thrombophlebitis of the lower extremities; other causes include occult malignant neoplasms, local trauma, and parenteral drug abuse. In a substantial number of patients the condition may be idiopathic.
The common clinical presentation of superficial thrombophlebitis is local pain, erythema, and induration, with tenderness of the thrombosed vein. When thrombophlebitis occurs below the knee, management consists of bed rest, leg elevation, and local application of heat to the affected veins. The disorder is usually self-limiting as obliteration of the affected part of the superficial venous system precludes subsequent attacks. The risk of thromboembolism is minimal and anticoagulation is not indicated. When thrombophlebitis extends above the knee, embolization may occur: such patients should be closely observed for cephalad progression of thrombus. Anticoagulation to prevent thromboembolism is indicated if the response to conservative management is poor.
Deep venous thrombosis is serious since the thrombus is much more likely to embolize to the lungs: when the thrombosis is proximal to the calf, there is a 50 per cent likelihood of pulmonary embolism, and up to 30 per cent of thrombi isolated to the calf veins embolize to the lungs. As many as 40 to 50 per cent of patients with deep venous thrombosis who develop pulmonary embolism have no symptoms of deep venous disease, causing a delay in the administration of appropriate prophylactic and therapeutic measures.
In patients who develop symptoms, mild oedema, superficial venous dilatation, and pain in the calf are usually present. Palpation of the calf may disclose tenderness and occasionally a thrombosed vein can be felt at any site from the plantar aspect of the foot to the femoral triangle in the groin. A thrombosed vein is usually best identified by palpation in the popliteal space. Homans' sign (tenderness and tightness in the calf with hyperextension of the foot) provides further evidence of thrombosis, but it may be present with any type of calf muscle irritation and is not pathognomonic for thrombotic disease.
Most forms of deep venous thrombosis involve the popliteal vein and its tributaries, but occasionally the thrombosis extends proximally to the femoral or iliac veins. Swelling and pain in the distal thigh are more prominent if femoral vein thrombosis is present, but these signs may be absent. Phlegmasia caerulea dolens is the condition found when ileofemoral thrombosis is associated with massive swelling of the entire extremity to the inguinal ligament, severe pain, tenderness, and cyanosis. Ileofemoral arterial thrombosis with spasm is frequently present and is characterized by a pale cool extremity with diminished or absent pulses. Disease confined to the popliteal vein and its tributaries may be occult or confused with other conditions such as rupture of the gastrocnemius muscle or disorders involving the knee, particularly a ruptured Baker's cyst. It is therefore important to confirm objectively the presence of suspected deep venous thrombosis.
Diagnostic tests
The most specific test for confirmation of the diagnosis of deep venous thrombosis is venography, in which contrast medium is injected into a vein on the dorsum of the foot to demonstrate the venous drainage through the popliteal, femoral, and iliac veins. A normal result nearly always excludes the presence of venous thrombosis. However, venography may be complicated by venous thrombosis and extravasation of contrast media produces perivasculitis, cellulitis, and occasionally ulceration of the skin. Patients are, therefore, often initially screened with a non-invasive technique and undergo venography if the diagnosis remains in doubt. Venography using radioisotopes instead of contrast medium avoids complications, but although results with this technique are improving it is not in widespread use.
Real-time B-mode ultrasonic imaging combined with Doppler ultrasound (duplex scanning) is a practical, non-invasive method of assessment of blood flow in veins and valve cusp movement, and can differentiate between acute and chronic thrombosis. All of the major deep veins of the lower limb can be assessed; however, it cannot exclude the presence of thrombi in small veins and is less accurate in demonstrating thrombotic disease in the calf. With experience, it is both accurate and relatively inexpensive and can be used in patients requiring reassessment. Plethysmography is another non-invasive technique which is useful in the diagnosis of deep venous thrombosis. A calf plethysmograph measures volume changes and may detect the oedematous changes associated with thrombus formation. Like duplex scanning, plethysmography is helpful in demonstrating proximal thrombotic disease than that occurring in the calf.
Intravenous administration of radioactive fibrinogen is another sensitive non-invasive technique used to diagnose deep venous thrombosis. Following intravenous injection of fibrinogen, the legs are scanned with a gamma-camera to detect the developing thrombus, which incorporates radioactive fibrinogen. This test is particularly accurate for detecting thrombosis in the calf, but high background radiation from the pelvic bones and urinary bladder means that it is not useful in assessing veins in the upper thigh. Any inflammatory condition also causes increased radioactivity, and results are not available for at least 12 h. Radioactive scanning of the lower extremities demonstrates deep venous thrombosis in as many as 54 per cent of patients following surgical treatment of a fractured hip, 50 per cent of patients following prostatectomy, and 28 per cent of general surgical patients over the age of 40. Scanning following injection of I labelled platelets may also be useful in the diagnosis of deep venous thrombosis.
Magnetic resonance imaging (MRI) is a reliable method of diagnosing venous thrombosis and can demonstrate thrombi in the pelvic veins. MRI does not require the administration of intravenous contrast agents and can be used safely in patients with allergies to dye or with impaired renal function.
Treatment
Anticoagulation prevents the propagation of the original thrombus and the development of new thrombi while the existing thrombus is lysed by naturally occurring fibrinolysis. Intravenous heparin has rapid action and can be discontinued, or its effects reversed rapidly with protamine sulphate, to decrease the possibility of bleeding complications. Subcutaneous heparin is also effective.
Although anticoagulation is management of choice for most patients, fibrinolytics such as urokinase, streptokinase, and recombinant tissue plasminogen activator have been evaluated clinically. Fibrinolytics can completely lyse up to 70 per cent of existing thrombi, a feature that conventional anticoagulants lack. Among 108 patients with venographically verified deep venous thrombosis treated with streptokinase, total or partial thrombolysis was demonstrated angiographically in 60 (55.6 per cent). However, three died during treatment, all from pulmonary embolism, and six developed clinical signs suggestive of pulmonary embolism. Major bleeding was a complication in 16 patients (14.8 per cent), allergic reactions to streptokinase occurred in 23 patients, and one patient developed anaphylactic shock. Thus, although streptokinase was effective in the management of deep venous thrombosis, complications were significant and the routine use of fibrinolytic therapy has been prohibited by bleeding complications, especially in postoperative situations. In addition, lysis of thrombi more than 72 h old is reduced and fibrinolytics have no advantage over heparin in the prevention of recurrent venous thrombosis. Their role in managing deep venous thrombosis is therefore limited, although patients with extensive disease, such as in the iliofemoral system, may benefit from their use.
The surgical extraction of venous thrombi has been almost completely discontinued since the recurrence rate is high. Venous thrombectomy still has a role in the management of patients with extensive iliofemoral disease in which limb loss is imminent, such as in phlegmasia alba dolens.
FURTHER READING
1. Bell, P.R.F., Jamieson, C.W. and Ruckley, C.V. The Surgical Management of Vascular disease, W.B. Saunders, London, 1992.
2. Galland, R.B. and Clyne, C.A.C. Clinical problems in vascular surgery, Edward Arnold, London.
THEME: PULMONARY EMBOLISM
QUESTIONS FOR HOMEWORK:
1. Introduction
2. Classification
3. Pathophysiology
4. Clinical symptoms.
5. Diagnostics.
6. Differential diagnosis.
7. Treatment.
8. Postoperative complications.
PULMONARY EMBOLISM
Pulmonary embolism is a common and sometimes fatal complication of deep venous thrombosis. Although it is recognized in the postoperative period, most patients develop pulmonary embolism secondary to non-surgical disorders, including congestive heart failure, cerebrovascular accidents, chronic pulmonary disease, systemic infections, carcinomatosis, and many chronic disorders.
Emboli that prove fatal are generally 1.5 cm or more in diameter and 50 cm or more in length, and are often fragmented. The right pulmonary artery is more commonly affected than the left, and the lower lobes more often than the upper lobes. Emboli originate primarily in the systemic venous circulation—most arise in the iliac and femoral veins, but up to 20 per cent originate from other sources, including the inferior vena cava, the subclavian, axillary, and internal jugular veins, and occasionally the cavernous sinuses of the brain. Emboli due to neoplasms should also be considered in the differential diagnosis. Renal cell carcinoma metastasizes early in its clinical course, and direct extension to the renal vein and inferior vena cava may cause pulmonary embolism in 10 to 54 per cent of affected patients. Primary pulmonary neoplasms can also mimic pulmonary embolism. Cardiac tumours arising in the right atrium and right ventricle may be the site of extensive pulmonary emboli.
A primary feature of pulmonary blood flow is the low vascular resistance that enables flow in this bed to increase several-fold with minimal elevation of pulmonary arterial pressure. Physiological changes following pulmonary embolism are related to the size of the emboli and can be divided into those that produce microembolism (obstruction of terminal small arteries and arterioles) and those that produce macroembolism (occlusion of the large pulmonary vessels). Considerable reduction in the diameter of the main pulmonary artery and the primary branches (at least 50 per cent) is required to reduce pulmonary blood flow significantly or to produce pulmonary hypertension. Experimental thrombi of large diameter produced in the inferior vena cava and embolized to either the right or the left pulmonary artery 10 to 14 days after their formation produce minimal cardiovascular and respiratory responses. Occlusion of one pulmonary artery usually causes insignificant changes in the central venous pressure, right ventricular pressure, pulmonary arterial pressure, systemic arterial pressure, cardiac output, total oxygen consumption, and the electrocardiogram, despite occlusion of half of the pulmonary arterial circulation, provided the remaining lung is normal. If one lung is normal or nearly normal, removal of the opposite lung is relatively well tolerated: tidal volume and oxygen consumption at rest change only minimally. Similarly, ligation of one pulmonary artery or occlusion by an intraluminal balloon is accompanied by few cardiodynamic changes. During exercise similar occlusion causes an increase in pulmonary arterial pressure of only 12 to 50 per cent, while cardiac output may increase as much as three-fold. Such occlusion closely simulates the obstruction produced by large pulmonary emboli. It should be emphasized that these findings apply to healthy subjects: underlying cardiac or respiratory insufficiency alters this response appreciably. In patients with heart disease, exercise during unilateral occlusion of the right and left pulmonary artery by a balloon catheter produces a sharp elevation in pulmonary arterial pressure. Resection of less than one lung is followed by only minor changes in the pulmonary arterial pressure, whereas removal of a greater amount of pulmonary tissue is associated with elevated pulmonary arterial pressure.
Clearly, mechanical factors are the most important in determining the cardiodynamic effects of pulmonary embolism, but reflex effects may cause bronchoconstriction. Tachypnoea, pulmonary hypertension, and systemic hypotension have also been demonstrated following experimental embolization with small particles. This is probably not a common clinical problem, although it may occur after massive blood transfusion during which small emboli containing platelets, leucocytes, and fibrin may occlude the pulmonary microcirculation.
Clinical manifestations
A clinical diagnosis of pulmonary embolism may be difficult because of its similarity to a number of other cardiorespiratory disorders. Dyspnoea, chest pain, and haemoptysis are classic symptoms but are not sufficiently specific to establish a definite diagnosis. It should be emphasized that many patients have underlying cardiac disease, and dyspnoea and tachypnoea are the most frequent clinical findings. Accentuation of the pulmonary second sound is also common. Haemoptysis, pleural friction rub, gallop rhythm, hypotension, cyanosis, and chest splinting are present in no more than one-quarter of patients. Clinical evidence of venous thrombosis occurs in only one-third of patients.
Special examinations
In patients with acute pulmonary embolism and no other pulmonary disease, the plain chest radiograph is usually normal. Diminished pulmonary vascular marking at the site of embolism may be present (Westermark's sign). The ECG is not specific but significant changes can be confirmatory. No more than 10 to 20 per cent of patients subsequently proven to have pulmonary embolism show any alterations in the ECG, including disturbance of rhythm (atrial fibrillation, ectopic beats, heart block), enlargement of P waves, S-T segment depression, and T-wave inversion (particularly in leads III, AVF), and of these, a smaller number show diagnostic abnormalities. The most common abnormality is S-T segment depression due to myocardial ischaemia, reduced cardiac output, and low systemic arterial pressures, as well as increased right ventricular pressure. Arterial blood gases are often normal, especially in young patients. Because clinical findings and routine examinations are non-specific, radioactive pulmonary scanning and pulmonary angiography are used to diagnose pulmonary embolism.
Radioactive pulmonary scanning
Radioisotope perfusion and ventilation pulmonary scans remain the most frequently employed technique in the diagnosis of pulmonary embolism. The method involves the detection of intravenously injected particles such as technetium 99 that become lodged in the pulmonary capillary bed.
The results of ventilation/perfusion scans are usually described in terms of the probability of embolism. High probability is indicated by segmental or greater perfusion defects with a normal ventilation scan (V/Q mismatch). Moderate probability is indicated by multiple subsegmental perfusion defects with a normal ventilation scan or segmental perfusion defects without a ventilation scan. Scans which are indeterminate show chronic obstructive pulmonary disease on the chest film or regions of perfusion scans, while subsegmental perfusion defects without a ventilation scan or matched perfusion and ventilation defects indicates a low probability of an embolism. High probability scans have a high positive predictive value for pulmonary embolism while low probability scans with low clinical suspicion are rarely associated with such emboli. Intermediate probability scans and low probability scans with high clinical suspicion are often associated with pulmonary embolism and indicate the need for arteriography to be performed.
Venograms or other non-invasive tests may be useful in establishing a definitive diagnosis of deep venous thrombosis, particularly in patients in whom the diagnosis is in doubt or when insertion of a vena caval umbrella is being considered.
The most definitive test for the diagnosis of pulmonary embolism is pulmonary arteriography. It is important that the appearance of the normal pulmonary angiogram is familiar in order that morphological and physiological changes can be appreciated. The arteries in the lower areas of the lung are normally larger because of a greater volume of pulmonary tissue. In most patients who survive the initial embolic episode the obstruction in the pulmonary arteries involves lobar or segmental branches. The defect should remain constant on several successive films in the series, and the flow may be sluggish, shown by a small pool of contrast medium that may persist in the artery above the obstruction after the venous phase of the angiogram. When pulmonary arteriography is performed later in the course of embolism, contrast medium may pass around the obstruction, causing delayed opacification of the artery distally. In some areas avascular segments resulting from unresolved thromboembolism may be seen. Oblique views of the pulmonary arteriogram should be obtained for maximal visualization and diagnostic accuracy. Pulmonary arteriography is safe when experienced radiologists use small diameter catheters and low osmolarity contrast agents.
MANAGEMENT
The importance of reaching an unequivocal diagnosis of pulmonary embolism before therapy is instituted warrants emphasis. The most reliable means of achieving absolute diagnosis are pulmonary scanning and arteriography. Because scanning is a simple, safe, and reliable technique, it is generally performed initially. If the pulmonary scan is to be used for definitive diagnosis, a concomitant plain chest film must show a normal pulmonary appearance in the area in which the scan demonstrates pulmonary arterial occlusion.
Prevention
Prophylaxis of deep venous thrombosis and pulmonary embolism is an important aspect of postoperative care. Nevertheless, no method or combination of methods completely prevents thromboembolism. Factors which reduce the risk include physical activity and elevation of the lower extremities for gravity drainage of venous return. Some consider compression of the legs by stockings or mechanical devices and prophylactic anticoagulation to be useful, but many disagree and these issues remain controversial. Early ambulation and resumption of physical activity after operation or bed rest for any reason has long been recommended.
Antiplatelet agents
Antiplatelet drugs play a role in the management of patients with thromboembolism. Non-steroidal anti-inflammatory drugs, including aspirin and dipyridamole, have also been shown to inhibit the platelet-release reaction secondary to ADP-induced platelet aggregation and adherence to collagen in vitro. Dipyridamole inhibits phosphodiesterase and raises intracellular cyclic AMP levels. The usual dose of 50 to 100 mg four times daily has no effect on platelet function, and it is usually administered in a combination with aspirin. Aspirin reduces the incidence of thrombosis from 20.4 per cent in controls to 12.5 per cent. In one study 1.2 g of aspirin daily was shown to be as effective as warfarin in preventing clinically diagnosed venous thrombosis and pulmonary embolism in a group of patients undergoing elective hip replacement. The incidence in the group receiving aspirin was 9 per cent, compared with 35 per cent in a previously studied control group. Studies of the overall effectiveness of antiplatelet drugs in the prevention of venous thrombosis and pulmonary embolism are continuing, because absolute certainty concerning their use has not yet been established. Dextran has also been used as an antiplatelet drug to prevent deep venous thrombosis.
Position and compression devices
Elevation of the legs with flexion of the knee as depicted in causes a rapid runoff of the blood in the veins of the leg and thigh due to gravity. This is a simple, effective, and broadly applicable prophylactic measure. Pneumatic compression devices also decrease stasis and increase venous blood flow but are not widely used.
Prophylactic anticoagulation
While prophylactic anticoagulation may be beneficial, especially after trauma and in patients with orthopaedic disorders, the concept of low-dose heparin as a prophylactic measure remains controversial. The usual regimen is an initial dose of 5000 units subcutaneously, repeated every 8 to 12 h until the patient is fully ambulatory. Coagulation times are prolonged minimally, if at all, with a low risk of bleeding. The protection conferred, if any, may be due to the potentiation of a naturally occurring plasma inhibitor of activated factor X. A large number of trials with low-dose heparin given to surgical patients postoperatively have used I labelled fibrinogen scanning or venography, or both, for the demonstration of development of venous thrombosis. With some exceptions, these studies have generally indicated a decrease in the occurrence of deep venous thrombosis, compared with that in controls. The efficacy of low-dose heparin in the prevention of postoperative pulmonary embolism is less obvious. The most frequently quoted of these studies is a multicentre clinical trial involving more than 4000 patients. Another randomized study of a series of patients aged over 40 years undergoing intraperitoneal procedures under general anaesthesia lasting more than 30 min found that the incidence of calf vein thrombosis was reduced by low-dose heparin but that the incidence of proximal vein thrombosis and pulmonary emboli, which were detected by chest films, pulmonary function tests, and perfusion scanning, was not reduced.
Although low-dose heparin continues to be recommended by some to prevent thromboembolism, it is not frequently used. It appears to have limited value after prostatectomy, after myocardial infarction, and in major orthopaedic procedures, particularly repair of femoral fractures and reconstructive surgery of the hip and knee. Low-dose heparin prophylaxis is also inadequate for patients with an active thrombotic process. Finally, it may lead to heparin sensitivity and cause disseminated intravascular coagulation, a condition in which platelets aggregate into thrombi and which may result in gangrene of the extremities.
Anticoagulation therapy
Anticoagulation with heparin is the standard treatment for acute thrombosis and pulmonary embolism. Heparin is administered intravenously at an initial dose of 5000 to 10 000 units followed by constant infusion at a rate sufficient to raise the activated partial thromboplastin time to 1.5 to 2 times the control value, usually 1000 units/h. Since heparin is excreted mainly in the urine, the patient's renal status must be monitored. The duration of heparin therapy varies, but 5 to 10 days is generally appropriate as this is the time usually required for thrombi to become adherent to the venous wall. Oral coumarin therapy, begun several days before cessation of heparin therapy, allows adequate prolongation of the prothrombin time.
The major complication associated with heparin therapy is bleeding, especially from surgical sites and into the retroperitoneum. Aspirin therapy, poor platelet function, and intramuscular injections may contribute to the risk of significant bleeding. Although heparin anticoagulation can be rapidly reversed by the administration of protamine sulphate, this is not usually necessary as reduction or cessation of heparin often stops the bleeding. Delayed haemorrhage may occur in patients with recent prosthetic arterial grafts. Continuous lysis and resorption of old thrombus and its replacement with new thrombus occurs in suture lines until they are sealed by regeneration of new intima. Haemorrhage may occur up to 1 month after the placement of arterial grafts in patients maintained on heparin therapy. Contraindications to heparin therapy include internal bleeding, intracranial neoplasm, recent cranial surgery, trauma, or haemorrhagic stroke.
Thrombocytopenia occurs in about 10 per cent of heparin recipients: this may be severe, accompanied by intravascular platelet aggregation and arterial thrombosis. Recognition of this complication is critical since cessation of heparin can reverse this syndrome and may be life-saving. Commercial heparin preparations are heterogeneous and only about 20 per cent of the infused material has anticoagulant activity. Low molecular weight heparin fractions that retain anticoagulant activity are the treatment of choice in patients with heparin-dependent thrombocytopenia who require additional therapy. These fractions do not interact with platelets and may not cause thrombocytopenia. Administration of heparin for longer than 2 months is also associated with a risk of osteoporosis.
The coumarin anticoagulants (coumadin and warfarin), which prevent the reduction of vitamin K in the liver and induce a state analogous to vitamin K deficiency, are used to prevent the recurrence of venous thrombosis and pulmonary embolism. The average loading dose of coumadin is 15 to 30 mg on the first day and 10 to 20 mg on the second day. The maximal effect is usually reached in 36 to 48 h, and the average daily maintenance dose is usually between 5 and 10 mg (range 2-20 mg). Anticoagulation is easily achieved by administration of coumadin once daily, adjusting the dose until the desired prolongation of the prothrombin time is achieved.
The prothrombin time should be monitored regularly in patients receiving oral anticoagulants. Despite the most careful management, prothrombin time may fluctuate, and drugs that alter anticoagulant metabolism or degradation in the liver or which compete with albumin binding sites can increase or decrease their potency.
There is a direct relationship between the duration of anticoagulation and the risk of recurrent thrombosis. Although recommendations vary, most patients with a single uncomplicated thromboembolic event have maximal benefit after 3 to 6 months of anticoagulation. About 10 per cent of patients treated with an oral anticoagulant for 1 year have a serous complication requiring medical supervision and 0.5 to 1 per cent have a fatal haemorrhagic event, despite careful medical management. The anticoagulation effects of coumadin can be reversed by infusion of fresh frozen plasma or by the administration of vitamin K. In many patients reduction or omission of several doses improves haemostasis and stops haemorrhage. Despite the risk of bleeding, patients with prosthetic heart valves, severe mitral stenosis, cardiomyopathy, chronic congestive heart failure, recurrent or persistent atrial fibrillation, and an inherited prethrombotic disorder may require life-long anticoagulation.
Fibrinolytic therapy
Activators of the fibrinolytic system are frequently used to accelerate lysis of thrombi. These agents are either naturally occurring products or chemically modified derivatives, and they differ with respect to fibrin specificity and complications. Current indications for fibrinolytic therapy include patients with massive pulmonary embolism complicated by hypotension, severe hypoxaemia, and right heart strain or failure. In addition, fibrinolytic agents have been successfully administered to patients with extensive iliofemoral thrombophlebitis. While such therapy often resolves venous thrombi, there is no firm evidence that lytic therapy reduces the incidence of long-term complications. Fibrinolytic therapy may be of benefit in patients with thrombosis of the axillary vein, which does not respond well to conventional anticoagulation.
Two thrombolytic agents, streptokinase and urokinase, have been studied extensively. Both act by transforming plasminogen to plasmin but cannot discriminate between free and fibrin-bound plasminogen. Streptokinase is a soluble product of the metabolism of Streptococcus pyogenes (Lancefield Group A) which indirectly activates plasminogen. Patients who have suffered previous streptococcal infections may be allergic to streptokinase, clinically manifest as toxic reactions such as pyrexia, dyspnoea, tachycardia, and anaphylaxis. Urokinase is a product of renal epithelial and tubular cells which directly activates plasminogen. In a national co-operative study, urokinase combined with heparin therapy, compared with heparin alone, significantly accelerated the resolution of pulmonary thromboemboli at 24 h, as shown by pulmonary arteriograms, pulmonary scans, and right-sided heart pressure measurement. However, no significant differences in the recurrence rate of pulmonary embolism or in 2-week mortality were noted. Bleeding was a common complication, occurring in 45 per cent of patients receiving urokinase and heparin, compared with 27 per cent of those given heparin alone.
In a study of the long-term effects of thrombolytic treatment of acute and massive embolism, seven patients underwent pulmonary angiography with pressure measurements before and after intrapulmonary infusion of urokinase (average dose, 1724 units/kg.h) and heparin (average dose 17 units/kg.h). The treatment was monitored by daily measurements of pulmonary arterial pressure and was continued until normal pressure was achieved (an average of 6 days). Pulmonary angiograms showed massive obstruction before therapy, with improvement occurring within 6 days after treatment. The mean pulmonary arterial pressure declined from an average of 37 plusmin; 9 to 13 mmHg after 6 days and to 15 plusmin; 3 mmHg after 15 months. No recurrence of pulmonary embolism was observed. Mean pulmonary arterial pressure and total pulmonary resistance remained within normal limits in six of seven patients, at rest and during bicycle exercise in the supine position. All patients showed clinical signs of deep venous thrombosis early after treatment. Fifteen months later, four patients had normal deep veins, and three had phlebographic signs of old thrombosis. Normal pulmonary arteriograms were obtained in six of seven patients. The reserve capacity of the pulmonary vasculature assessed during heavy exercise was normal.
Streptokinase is usually given with a loading dose of 250000: this may need to be repeated since patients may have antistreptococcal antibodies. Urokinase is given with a loading dose of 4400 units/kg body weight, administered over 10 to 30 min. A systemic lytic state develops with a decrease in fibrinogen levels, prolongation of the thrombin time, and prolongation of the euglobin lysis time. After the initial loading dose, 100000 units of streptokinase or 4400 units of urokinase/kg body weight are administered hourly for 24 to 72 h. The lytic state is reversed by discontinuing the enzyme. Heparin therapy can be started after 6 h and is continued for 7 to 10 days. Fibrinolytic therapy should be initiated as soon as possible after the onset of thrombosis or embolism. The systemic fibrinolysis associated with the fibrinolytic agents may cause haemorrhage since essential haemostatic plugs are attached as well as pathological thrombi. Lytic therapy is therefore not recommended for patients with recent surgery or those with a history of a neurological lesion, gastrointestinal bleeding, or hypertension.
Recombinant tissue plasminogen activator is now available for general use. In one study, a group of patients with angiographically documented pulmonary embolism, all of whom had segmental or proximal pulmonary arterial obstruction within 5 days of the onset of symptoms or signs, were treated with 50 mg of recombinant tissue plasminogen activator every 2 h followed by an additional 40 mg every 4 h if required. Thirty-four of the 36 patients had angiographic evidence of thrombolysis by 6 h: clot lysis was slight in four, moderate in six, and marked in 24. Fibrinogen levels decreased by 30 per cent at 2 h and by 38 per cent at 6 h, with only two major complications. Infusion of recombinant tissue plasminogen activator via the artery does not offer significant benefit over administration by the intravenous route. Firm contraindications to the use of thrombolytic therapy include internal bleeding (recent or active), recent neurosurgery, insertion of an arterial prosthetic graft, cranial trauma, and a history of haemorrhagic cerebrovascular accident. Relative contraindications include a recent surgical procedure (within 7-10 days), cardiopulmonary resuscitation (within 7-10 days), or the presence of a coagulopathy.
Surgical management
While anticoagulant therapy of pulmonary embolism is usually successful, it may fail, and in this event the need for surgical management should be reviewed on an individual basis. Venous thrombectomy was previously recommended but is now rarely performed because of the high incidence of recurrent postoperative thrombosis. The presence of phlegmasia caerulea dolens with secondary arterial spasm is a rare indication for thrombectomy. Although thrombosis may recur in such patients, the venous lumen may remain patent for long enough to relieve the arterial spasm and prevent gangrene developing.
Although vena caval interruption was previously recommended for selected patients with pulmonary embolism, it is seldom performed today. A stainless steel umbrella designed by Greenfield and Michna can be inserted under local anaesthesia through the femoral or jugular vein. With this device a filter is fixed to the wall of the inferior vena cava by hooks. Complications include distal migration to the bifurcation of the inferior vena cava, protrusion of the struts through the caval wall, formation of thrombus on the filter, misplacement of the device, retroperitoneal haemorrhage, perforation of the duodenum or ureter, and development of a thrombus proximal to the umbrella, producing emboli. The filter may also migrate into the iliac vein, renal vein, right atrium, right ventricle, or pulmonary artery, and such migration is occasionally fatal. The filter may also stimulate distal thrombosis in the vena cava and late occlusion may occur. Other filter types used are the Amplatz, Gunther, and birds nest.
Pulmonary embolectomy
In 1908, Trendelenburg performed the first pulmonary embolectomy. He treated three patients with this procedure, the longest survival time being 36 h. In 1924, Kirschner performed the first successful pulmonary embolectomy associated with long-term survival. The first successful pulmonary embolectomy performed using extracorporeal circulation was reported in 1961: this is currently the preferred technique, since it permits continuous oxygenation of the body while the emboli are safely removed from the pulmonary arteries.
Persistent and refractory hypotension despite maximal resuscitation is the primary indication for pulmonary embolectomy, especially in a patient with massive embolism clearly documented by either a pulmonary scan or pulmonary arteriogram. Treatment includes systemic heparinization and the administration of vasopressors, inotropic agents, and endotracheal oxygen. The primary management should be by this approach, since many patients previously thought to require embolectomy respond favourably with intensive resuscitation. Usually, 1 or 2 h may be spent attempting to restore acceptable cardiopulmonary function, unless the clinical situation is desperate. An appropriate blood pressure should be maintained and embolectomy may be postponed, especially if renal and cerebral function is acceptable.
If pulmonary embolectomy is indicated, it is usually performed using cardiopulmonary bypass. A median sternotomy is made for exposure of the pulmonary artery. The main pulmonary artery is usually found to be free of emboli, although partial obstruction may be present. The emboli are removed from the right and left pulmonary arteries and from their major branches. Smaller emboli may be removed by passage of a Fogarty catheter and irrigation with saline. The pulmonary artery is closed and cardiopulmonary bypass is gradually discontinued, allowing the heart and lungs to resume normal function.
Patients with acute and severe cardiopulmonary collapse can be supported by partial cardiopulmonary bypass by a circuit from femoral vein to femoral artery for immediate resuscitation. If extracorporeal circulation is not available, a right or left thoracotomy with exposure of the most severely involved pulmonary artery can be performed, the side of predominant occlusion being determined by a scan or arteriogram. An anterior thoracotomy is appropriate for exposure of either the right or left pulmonary artery, which can be opened distally for removal of emboli while normal circulation to the opposite lung is maintained. A serious complication which may follow pulmonary embolectomy is massive endobronchial haemorrhage. Successful management involves endotracheal intubation for selective collapse of the lung and entrapment of the haemorrhage into the involved lung. Reperfusion pulmonary oedema may occur after pulmonary artery thromboendarterectomy, and prolonged mechanical ventilation is often required. The syndrome is a cause of postoperative hypoxaemia with local pulmonary infiltrate.
Chronic pulmonary emboli
Most pulmonary emboli eventually resolve as a result of the action of the natural fibrinolytic systems. In a small number of patients, however, these emboli gradually accumulate in the pulmonary arterial system owing to inadequate fibrinolysis or recurrent episodes of embolism. Patients with chronic pulmonary emboli have a history of exertional dyspnoea progressing to severe respiratory insufficiency over several months or years. They may also complain of recurrent episodes of thrombophlebitis, haemoptysis due to the presence of large bronchial collaterals, and chest pain. Physical findings include signs of severe pulmonary hypertension, often combined with evidence of right ventricular failure; this may be manifested as a increased pulmonary second sound, a systolic murmur, hepatomegaly, and a S3 or S4 gallop. Medical management is usually unsatisfactory, and these patients have a poor prognosis.
Chest radiographs show a dilated pulmonary artery and oligaemic pulmonary fields in approximately half of these patients. Right ventricular enlargement is present in two-thirds and pleural effusion in approximately one-third of patients. Analysis of arterial blood gases in patients breathing room air reveals evidence of severe respiratory insufficiency, with hypoxaemia and arterial oxygen tension values of 55 to 60 mmHg and an arterial carbon dioxide tension of approximately 30 mmHg. The electrocardiogram is usually suggestive of chronic cor pulmonale, including right-axis deviation and right ventricular hypertrophy. Peripheral venography or MRI demonstrates venous thrombosis and indicates the source of the emboli.
Ventilation and perfusion radionuclide scans are consistent with the presence of pulmonary emboli, and perfusion defects correspond to oligaemic regions of the plain chest film and pulmonary arteriogram. Perfusion defects are usually noted bilaterally. Pulmonary arteriography allows documentation of emboli, determination of anatomical distribution of emboli, and recording of pulmonary artery pressure: the natural history is related to the magnitude of pulmonary arterial hypertension. If the mean pulmonary artery pressure is more than 30 mmHg, survival at 5 years is only 30 per cent, while only 10 per cent of those with mean pressure greater than 50 mmHg are alive at 5 years. Arteriography usually shows emboli in both lungs, with 55 to 75 per cent of the total pulmonary blood flow obstructed. Further preoperative studies include a thoracic aortogram with selective bronchial arteriography to demonstrate dilated and tortuous bronchial vessels. The bronchial circulation is often considerably dilated and communicates by collaterals with the distal pulmonary arteries.
Surgical management
Embolectomy may be performed on one or both pulmonary arteries. Either a right or left anterior thoracotomy can be undertaken when there is proximal occlusion of one vessel. Patients with bilateral pulmonary emboli, or with embolus of the main pulmonary artery, generally requires extracorporeal circulation during the procedure. These emboli are firmly attached to the artery wall and great care is required in the dissection. All distal emboli should be removed until there is adequate back-bleeding of bright red arterialized blood. Satisfactory distal back-bleeding can usually be predicted in advance from the information gained by selective injection of the bronchial arteries.
Postoperative complications include right ventricular failure in patients with long-standing cor pulmonale and pulmonary hypertension, haemorrhagic lung syndrome, which can be managed successfully by tracheal intubation with a dual lumen catheter and balloon occlusion of the affected lung, and phrenic nerve paresis, usually as a result of topical hypothermia. Psychiatric disturbances may also occur and are usually transient.
Embolectomy for chronic pulmonary embolism generally decrease pulmonary artery pressures and increases toward normal. In patients with proximal pulmonary arterial obstruction pulmonary embolectomy is likely to produce relief of respiratory insufficiency, a reduction in pulmonary hypertension, and an improvement of right-sided heart failure.
TESTS
1. Earliest sign of deep vein thrombosis is-
a) Calf tenderness
b) Rise in temperature
c) Swelling of calf muscle
d) Roman's sign
2. White leg is due to –
a) Femoral vein thrombosis and lymphatic obstruction
b) Deep femoral vein thrombosis
c) Lymphatic obstruction only
d) None of the above
3. All of the following are seen in deep vein thrombi except -
a) Pain
b) Discolouration
c) Swelling
d) Claudication
4. Best method for diagnosis of deep vein thrombosis is –
a) Doppler examination
b) Plethysmography
c) Contrast phlebography
d) I 131 Fibrinogen studies
5. Commonest complication varicose vein stripping is –
a) Thromboembolism
b) Hemorrhage
c) Ecchymosis
d) Infection
6. Investigation of choice for diagnosis of deep vein thrombosis
a) Venogram
b) Doppler
c) Isotope scan
d) Homans sign
7. Which is not used in treatment of superficial venous thrombosis –
a) Immediate anticoagulation
b) Rest and elevation
c) Analgesics
d) Treat assosiated malignancy
8. Most common complication of varicose vein stripping is –
a) Infection
b) Haemorrhage
c) Ecchymosis
d) Thromboembolism
9. What is acceptable in the management of femoral vein thrombosis -
a) Bed rest and spiral elastic bandages
b) A venogram
c) Thrombectomy
d) Embolectomy
e) A mobin udin umbrella inserted into the vein
10. Operations for varicose veins are best accomplished by –
a) Stripping
b) Multiple subcutaneos ligatures
c) Subfascial ligatures
d) Division and ligation at the superficial venous system
11. A 60-years old male has been operated for carcinoma of caecum and right hemicolectomy has been done.
On the fourth post-operative day, the patient develops fever and pain in the legs. The most important clinical entity one should look for is –
a) Urinary tract infection
b) Intravenous line infection
c) Chest infection
d) Deep vein thrombosis
12. All of following may be predisposing factors for deep vein thrombosis except –
a) Oral contraceptives
b) Nephrotic syndrome
c) Sickle cell anemia
d) Thrombocytosis
13. The duration of heparin therapy in deep vein thrombosis is
a) 7- 10 days
b) 15-20 days
c) 3-4 days
d) 1 month
14. Most accurate & non invasive method for diagnosing deep vein thrombosis –
a) Doppler duplex
b) Plethysmography
c) Radioactive labelled fibrinogen
d) Angiography
15. Most common site for venous thrombosis -
a) Popliteal vein
b) Soleal vein
c) Femoral vein
d) Internal iliac vein
16. Warfarin is stppped … days prior to surgery -
a) 1-3
b) 3-5
c) 6-8
d) 8-10
17. Deep vein thrombosis is caused by all except -
a) Lower limb trauma
b) Hip and pelvic surgery
c) Subungual melanoma
d) Cushing's syndrome
18. The deficiency of all of the following factors increases the incidence of thrombus formation except -
a) Lipoprotein A
b) Protein - C
c) Anti - thrombin III
d) Protein – S
19. The most common vein to get thrombosed is the
a) Long saphenous
b) Short saphenous
c) Both
d) Posterior tibial
20.Brodie -Trendlenburg test demonstrates-
a) Mid - thigh perforation
b) Deep vein thrombosis
c) Sapheno — femoral incompetence
d) Calf perforators
21. An obese patient develops acute oedematous limb following a Pelvic surgery. Deep vein thrombosis is suspected . The most useful investigation in this case would be –
a) Doppler imaging
b) Fibrinogen uptake
c) Venography
d) Plethysmography
22. In a 40 years old male thrombus in the common femoral artery is because of –
a) Atheroma
b) Thrombangits obliterans
c) Reynauds disease
d) Abdominal mass
23. All are true about Embolic Arterial occlusion except -
a) No previous history
b) Muscles are unaffected
c) Pulse is absent
d) Anaesthesia is present
24. Treatment of acute femoral embolus is –
a) Warfarin
b) Heparin
c) Immediate embolectomy
d) Embolectomy after 5 days bed rest
25. Intermittent claudication is caused by –
a) Venous occlusion
b) Arteria insufficiency
c) Neural compression
d) Muscular dystrophy
26.Management of a cause of iliac artery embolism requires-
a) Embolectomy
b) Injection of vasodilators
c) Hypotensive therapy
d) Sympathectomy
27. Intermittent claudication at the level of the hip indicates-
a) Popliteal artery occlusion
b) Bilate iliac artery occlusion
c) Common femoral occlusion
d) superficial femoral artery occlusion
28. Most common cause of death in patients with Burger's disease is -
a) Gangrena
b) Pulmonary embolism
c) Myocardial infarction
d) Carcinoma lung
ANSWERS
A
B
D
A
C
B
A
C
A,C
D
D
C
A
A
None
C
C
A
D
C
A
A
B
C
B
A
B
C
ГОУ ВПО «Смоленская государственная медицинская академия
Федерального агентства по здравоохранению и социальному развитию»
МЕТОДИЧЕСКИЕ УКАЗАНИЯ ДЛЯ СТУДЕНТОВ
ПО ДИСЦИПЛИНЕ хирургические болезни
ENDOSCOPIC SURGERY
Составитель доц. А.Ю.Некрасов
Методические указания утверждены на методическом совещании кафедры госпитальной хирургии (протокол № 2 от 6 октября 2008 г.)
Зав. кафедрой______________(проф. С.А.Касумьян)
2008 г.
INTRODUCTION
Surgeons have always wanted to look inside the human body but rigid and primitive instruments, poor illumination, and inadequate anaesthesia originally confined such procedures to the natural orifices. General anaesthesia and, at the end of the nineteenth century, the introduction of electric light and glass telescopes first made cystoscopy feasible and later bronchoscopy and laparoscopy. An angled telescope allowed the surgeon to see around a corner but the continuous curves of the bowel remained a barrier to further progress in gastrointestinal endoscopy until the development of flexible glass fibres. Gynaecologists were the first to appreciate the advantages of laparoscopy. Parallel developments in instrumentation and illumination soon meant that some therapeutic procedures could be performed although there were limitations because the operator was the only one who could see what he was doing and one of his hands was occupied holding the endoscope itself. Nevertheless laparoscopic sterilization and transurethral resection of the prostate have been standard operations for many years.
Minimally invasive or endoscopic surgery for a much wider range of operations has become possible as a result of the development of miniature television cameras which can be attached to any suitable telescope. The cameras are unobtrusive, light in weight, and provide a perfect and magnified colour image of the operative field on a television monitor. The surgeon has both hands free to manipulate instruments and any number of assistants can watch what is being done on separate monitors and operate as well. New instruments have been developed to overcome the limitations on access and movement within the body cavities and new ways of performing old and well established operations have also been devised. In that respect this chapter will be out of date by the time it is published but even so the basic principles of endoscopic surgery are already well established.
LAPAROSCOPY
The peritoneal cavity of a dog was examined in 1902 by Kelling, using air insufflation and the insertion of a cystoscope through the abdominal wall. The first clinical use was described in 1912 by Jacobaeus, although it was a further decade before a purpose-built scope was in use by Kalk (1929) and the era of modern-day laparoscopy (peritoneoscopy) began.
PROCEDURE (DIAGNOSTIC LAPAROSCOPY)
The patient is positioned supine on the operating table. A general anaesthetic with muscle relaxation is usually preferred, but it is possible to use local anaesthetic and sedation with intravenous benzodiazepines. The Verres needle is introduced via a stab incision. This is usually subumbilical in position, but the presence of scars may influence the precise location. The needle contains a spring-loaded blunt probe, and compression of the spring against the skin retracts the probe to expose the needle. Damage to intra-abdominal viscera can be minimized by holding up the anterior abdominal wall with one hand while inserting the needle with the other. When the needle has passed through the abdominal wall the resistance falls and the spring pushes forwards the probe covering the needle. Free flow of normal saline solution through the needle confirms that the linea alba and peritoneum have been punctured. The abdomen is then insufflated with carbon dioxide, using approximately 2 to 3 litres for an adult. During insufflation, the intra-abdominal pressure should not exceed 15 mmHg. The Verres needle is then withdrawn and the incision is enlarged to accommodate the laparoscope trocar, which is pushed down and back into the pelvis. The end- or side-view telescope is then inserted and laparoscopy commenced. Biopsy forceps and a palpating probe can be used in other, suitably placed stab incisions through the anterior abdominal wall. This allows the peritoneal contents to be inspected. Throughout the procedure, carbon dioxide is continually insufflated at low pressure.
CONTRAINDICATIONS
There are few absolute contraindications to the procedure, but certain conditions should alert the surgeon to potential problems. Multiple scars make introduction of the scope hazardous, and adhesions from repeated abdominal procedures may hinder the view within the peritoneum. Abdominal wall sepsis may introduce intraperitoneal infection. The procedure is not tolerated well in patients with severe pulmonary or cardiac problems, due to the intra-abdominal distension. Bleeding diatheses may result in body wall or intraperitoneal bleeding.
COMPLICATIONS
To minimize complications, laparoscopy is a procedure best performed by surgeons experienced in the technique, in an operating theatre equipped with the facilities to proceed to a laparotomy if necessary. Minor complications include abdominal wall bruising, subcutaneous emphysema, the development of a wound infection/hernia, and postoperative shoulder pain. Other complications are related to accidental visceral damage and bleeding from vessel injury. These problems should be noted at the time of laparoscopy and dealt with by prompt laparotomy if necessary. Mortality rates of 0.03 to 0.1 per cent are reported.
INDICATIONS
Acute
In patients with localized peritonism, diagnostic laparoscopy is most commonly used in the management of patients with acute right iliac fossa pain, in an attempt to reduce the incidence of negative surgical explorations for acute appendicitis, especially in young women. With the aid of a palpating probe inserted through the anterior abdominal wall of the right iliac fossa, the surrounding ileum and omentum may be manipulated away in order to see the appendix. In the case of a retrocaecal or retroileal appendix, it may be impossible to visualize the target organ, but other signs of acute inflammation may be noted. Alternatively, other causes of right iliac fossa pain may be apparent, and, if these require surgery, an appropriate incision can be made.
The role of diagnostic laparoscopy in the management of the patient with abdominal trauma is in conjunction with imaging techniques (CT and ultrasound scanning) and peritoneal lavage. The relative importance of each is not established clearly, although aggressive use of laparoscopy in this clinical situation may reduce the number of unnecessary laparotomies performed for minimal or moderate haemoperitoneum. The procedure can be performed in the accident and emergency department under local anaesthesia with intravenous sedation, and has been facilitated by the development of a (5 mm) laparoscope.
Elective
In the management of patients with undiagnosed abdominal pain, opinion is divided as to the benefits of laparoscopy. The role of laparoscopy is better established in the investigation of women with chronic pelvic pain, and is frequently performed by gynaecologists.
In children with impalpable testes, laparoscopy can be used to visualize the internal ring and the testicular vessels. These are followed to locate the testis, which may then be classified as being abdominal, pelvic, canalicular (the lower pole entering the orifice of the internal ring), or absent. The laparoscopic findings may then influence the site of a subsequent incision or obviate the need for an exploration.
Other indications for elective laparoscopy in benign disease include the diagnosis of various liver conditions (by direct visualization with or without biopsy), and tuberculous peritonitis.
Laparoscopy is commonly used in the management of intra-abdominal malignancy. As a staging investigation, laparoscopy is a more sensitive and accurate means of detecting small liver metastases and peritoneal seedlings compared with CT and ultrasound scanning. Biopsy may be performed using a Menghini needle under direct vision, which minimizes the risk of inadvertent visceral trauma, and increases the positive histological yield compared with a percutaneous biopsy. Draining lymph nodes may also be seen and biopsied to exclude malignant involvement. The knowledge obtained by such procedures may avoid an unnecessary laparotomy if a palliative operation is not indicated.
SPECIFIC OPERATIONS
The development of endoscopic surgery can be compared in importance to the introduction of anaesthesia into surgical practice and the impact will be felt well into the next century. The enthusiasm for the endoscope now is in marked contrast to the rejection of the laparoscope in the past. It is true that gynaecologists have always used laparoscopy but few general surgeons embraced the technique, mostly because only a limited number of organs could be seen and not many abdominal operations are possible with one eye and one hand occupied with a laparoscope. It is the development of video equipment that has made the revolution possible.
It was also fortuitous and fortunate that the first operation to make endoscopic surgery a household word should have been cholecystectomy. The endoscopic operation is identical to an open procedure, it is not particularly difficult to do in most cases, and the benefits to the patient were, and still are, dramatic. There has been some evidence of overenthusiasm. A few obsolete operations have been resurrected because they are easy to do laparoscopically and a few serious complications have occurred but overall the advantages are obvious and patients themselves have been pressing to have an endoscopic operation where possible.
The intention in this section is to illustrate what is presently possible in detail for some operations and in outline for others. Many more endoscopic operations will have been described by the time this chapter is published and we shall leave the reader to discover more information either elsewhere in this book or in a specialist text.
The significant disadvantages of a major abdominal wound and the success of laparoscopic cholecystectomy has ensured the rapid development of endoscopic abdominal surgery. The retroperitoneal organs, with the exception of the kidney, are inaccessible at the moment and most of the operations, like cholecystectomy, are endoscopic adaptations of a conventional operation. A few procedures, for example hernia repair, explore new ideas and more of these can be expected.
Laparoscopic cholecystectomy
Laparoscopic cholecystectomy was first performed in 1987 by Phillipe Mouret in Lyons, France. The operation was immediately accepted by the surgical community and has been followed by a worldwide wave of enthusiasm for endoscopic surgery. The laparoscopic operation reduces the access required to an absolute minimum whilst at the same time achieving the same objective as a conventional open cholecystectomy.
Every patient who needs a cholecystectomy and is fit for a general anaesthetic should be suitable for the laparoscopic technique although pregnancy and portal hypertension might make the operation difficult. There are no absolute contraindications to a laparoscopic operation although the discovery of some unsuspected disease or problem during a preliminary laparoscopy may require a full laparotomy. Obesity is not a contraindication. The view of the operative field is just as good in a fat patient as it is in a thin one and the absence of an abdominal incision reduces the incidence of complications. Dense peritoneal adhesions, acute cholecystitis, severe chronic cholecystitis, and difficulty in identifying the anatomy in Calot's triangle can make continuing with a laparoscopic operation dangerous. Conversion to an open operation in these circumstances, which is necessary in about 5 per cent of patients, demonstrates good surgical judgement and must not be regarded as a failure.
Once the cystic duct and the cystic artery are divided the free edge of the lesser omentum falls away. The midcostal grasper is repositioned just above the cystic duct clip and it is pulled upwards and away from the liver to expose the plane between the liver and the gallbladder. The gallbladder is then dissected off the liver bed with scissors, diathermy, or a laser beam starting at the neck. All the bleeding points on the liver bed are coagulated as dissection proceeds until the gallbladder is only just attached to the liver at the fundus. Any bile or blood that has accumulated is sucked away and, if necessary, a drain is placed in the gallbladder bed before this final strand is divided.
Dissection at the fundus is often difficult and simply requires patience and constant changes of position. If the gallbladder is perforated, and this is preferable to entering the liver itself, the bile and as many stones as possible should be removed. Nevertheless stones are often left behind in the peritoneal cavity where, somewhat surprisingly, they very rarely cause complications.
Once the gallbladder is free the cystic duct is drawn into one of the 11 mm ports and the port and the gallbladder are withdrawn together through the abdominal wall. Usually the fundus of the gallbladder and any contained stones remain within the abdomen and they must be manipulated through the wound. Crushing any large stones, sucking away the bile or extending the incision are sometimes necessary and all of these are easier to do if the gallbladder is inside a plastic bag.
A final inspection of the abdomen is appropriate before all the carbon dioxide gas is allowed to escape and the cannulae are removed. We only suture the abdominal wall if a wound has been enlarged and we prefer to close the skin with adhesive tape rather than with sutures.
The best management of bile duct stones in this new endoscopic era is not yet clear. We prefer to remove any stones from the bile duct at an ERCP procedure before embarking on a laparoscopic operation but this may not be appropriate and, sometimes, is impossible.
Large stones, which tend to occur in older patients with grossly dilated bile ducts, always cause difficulty at endoscopy but they are relatively easy to remove at a laparoscopic exploration of the bile ducts. In young patients it is best not to divide the ampullary sphincter and the duct must be explored surgically. The choice lies between a conventional open operation or one of the newer laparoscopic techniques.
Stones can be extracted from the bile duct through the cystic duct if it is first dilated with a suitable balloon. Instruments, including a narrow cholangioscope, can then be passed into the bile duct and the stones retrieved or broken up under direct vision. Stones in the hepatic ducts are difficult to find, particularly if the cystic duct enters the common duct low down behind the pancreas. A fine tube can be left in the cystic duct at the end to act as a drain and to permit later cholangiography. The precise place of this technique in endoscopic practice is not yet clear.
Direct endoscopic exploration of the bile duct is quite straightforward. A forward oblique telescope gives the best view of the bile duct and a supraduodenal choledochotomy is easily made in the anterior surface of the duct with the diathermy hook and scissors. Stones can be washed out of the duct or removed with a balloon or a basket. The ducts are then inspected with a choledochoscope and any remaining stones are removed. At the end a t-tube is sutured into the bile duct.
If stones are discovered in the bile ducts unexpectedly during the course of a laparoscopic cholecystectomy the surgeon is left in something of a dilemma. Small stones can probably be safely left to pass spontaneously. Larger stones need to be removed either immediately and surgically or at a later ERCP.
All three choices have disadvantages and deciding between them depends on the findings in the individual patient, the equipment available, and the skill and experience of the surgeon. If stones are left behind in the ducts it is probably wise to leave a drain down to the cystic duct stump in case it leaks. An ERCP should be done within a day or two of operation to remove any residual stones and as soon as retained stones are discovered.
Inserting a cannula or the Veress needle can cause spectacular damage and invisible retroperitoneal aortic injury is one cause of an acute collapse during a laparoscopic operation. Such injuries are fortunately very rare.
Sudden severe haemorrhage is always a problem and it is best avoided in the first place. A torn cystic artery or a liver laceration are the common causes and haemorrhage from either can be difficult to stop. The usual general principles apply. Blood and clot are sucked or washed away and the best possible exposure is obtained whilst the surgeon applies local pressure to the bleeding area. The scrub nurse prepares the diathermy and a titanium clip applier and when everything is ready the pressure is removed and the bleeding point is identified and occluded. This is much more difficult to achieve with the limitations on movement in an endoscopic environment and there is sometimes no alternative to a laparotomy.
Every surgeon fears damaging a bile duct. In large published series the accident happens once in every 300 laparoscopic cholecystectomies. This is slightly more frequent than during an open operation and probably reflects the introduction of a new technique rather than a fundamental flaw in the operation. Great care should be taken to identify the anatomy correctly and to place any clips properly. It is very easy for a titanium clip to be placed so that the tips partially occlude the bile duct without the surgeon appreciating what has happened and any doubts about the anatomy should lead to an immediate laparotomy. The next most important aspect of a bile duct injury is that it should be recognized at the time. Late presentation of biliary damage is always associated with a poorer outcome. Exactly what is done depends upon the injury and when it is recognized but a laparotomy will usually be needed.
Reactionary haemorrhage is rare and generally requires a laparotomy although with increasing experience a repeat laparoscopy may be sufficient. Bile leaks from the cystic duct stump or the liver bed present with pain in the right upper quadrant, fever and a subhepatic collection on ultrasound. The bile is removed with a percutaneous drain and the leak is identified at an ERCP and is stopped by placing a stent in the bile duct. The drain remains until the drainage ceases and the stent is removed not less than 2 months later. Surprisingly a simple sphincterotomy will not allow the leak to close. A few patients present with a previously unidentified bile duct stone within a few months of their operation and the stone should be removed through an endoscopic sphincterotomy.
The mortality rate following a laparoscopic cholecystectomy is about 0.1 per cent and the complication rate is about 4 per cent. Both figures are much better than those for an open operation and the cosmetic benefits of four small incisions are important to many patients. At the moment about 1 in every 20 patients needs a laparotomy although we can expect this figure to improve as experience increases. Patients must be warned about this beforehand and sign an appropriate consent form.
Most patients are mobile and can eat and drink later the same day. Some patients are troubled by nausea and vomiting but very few need opiate analgesia. Any drain can be removed after about 12 h and most patients can then go home. The majority are back at work within 2 weeks.
Laparoscopic appendicectomy
Laparoscopic appendicectomy combines the advantages of diagnosis and treatment in one procedure. The usual preoperative preparation is necessary and prophylactic antibiotics are given on induction of general anaesthesia. The patient must consent to an open operation should it be needed.
The patient lies flat on the operating table and the bladder is emptied. After creating the pneumoperitoneum an 11 mm port and endoscope are placed through the umbilicus and the diagnosis is confirmed. Two further ports are needed on either side of the abdomen and should be placed in relation to the position of the appendix. An 11 mm port is placed in the right iliac fossa and a 5.5 mm port in the left iliac fossa.
Any local adhesions are gently divided and the tip of the appendix is grasped and drawn into the port in the right iliac fossa. The appendix mesentery is occluded with bipolar diathermy or a ligature around the appendicular artery and then divided. The base of the appendix is secured with a Roeder knot and then occluded beyond the ligature with bipolar diathermy. The appendix is divided across the burnt area and is removed through the right iliac fossa port. It is not necessary to bury the appendix stump. Free peritoneal fluid or pus can be sucked away and the peritoneal cavity washed although it is important not to flood infected fluid into the pelvis or the subphrenic spaces. It is easy to place a drain to the appendix stump if necessary and the pneumoperitoneum is then released and the ports removed.
Most patients have less pain from the wounds than after a conventional appendicectomy but the recovery time is only slightly improved. Many patients are toxic from the infection and take time to recover whatever method is used to remove the appendix.
The only complication specific to the laparoscopic procedure is a low incidence of reactionary haemorrhage as a result of failure to occlude the appendicular artery properly. Septic complications occur just as with any other appendicectomy although wound infection is less serious.
Laparoscopic colectomy
Laparoscopic colonic resection is already possible for the techniques involved are no different to those used in a laparoscopic cholecystectomy or appendicectomy. However, tying and dividing the mesentery can be very tedious and the resection may not include all the important lymph nodes. It also takes a long time and considerable skill to construct a hand-sewn intraperitoneal anastomosis and although stapling guns can help they are presently too expensive for routine use. So a complete laparoscopic colonic resection is not really a practical option at the moment although this situation is unlikely to persist as new technological developments are introduced.
Nevertheless endoscopic techniques can be useful during colonic resection in certain patients. Division of the mesentery under laparoscopic vision is fairly straightforward and the segment of bowel can be delivered through a small discreetly placed incision, the resection and anastomosis being completed outside the abdominal cavity. The bowel is returned to the abdomen and any necessary drains are placed before all the ports are withdrawn and the incision closed. This approach minimizes the trauma to the patient and overcomes the problem of removing the specimen from the abdominal cavity.
Once the ideas and the concepts of an endoscopic operation are accepted it takes very little imagination to transfer almost every operation to an endoscopic procedure. Indeed most abdominal operations have already been done laparoscopically somewhere in the world.
Operations around the stomach, such as fundoplication and pyloromyotomy in neonates, are relatively easy to do although there is little demand for vagotomy. Small bowel resections are mostly required during emergency operations and although endoscopic techniques are described most surgeons will be naturally hesitant to embark on such procedures at the moment. The same remarks apply to splenectomy. Liver resections are clearly impractical at present but it is easy to deroof a liver cyst with a diathermy hook and bleeding from a liver biopsy site can often be controlled with a laparoscopic technique, thus avoiding a laparotomy. In both instances it is quite possible to suck away blood or other fluids lying in the peritoneal cavity.
Emergency laparoscopic surgery is still being developed. A perforated anterior duodenal ulcer is easy to close with an omental patch through the laparoscope as any surgeon who has done a laparoscopic cholecystectomy will appreciate. Laparoscopy for intestinal obstruction is difficult because of the distension but it is possible to decompress the bowel and then to divide an adhesive band. Some reports have also suggested that laparoscopy and laparoscopic surgical techniques are useful in patients with abdominal trauma.
In some respects endoscopic surgery has not advanced so rapidly in gynaecology. This is partly because transcervical resection of the endometrium has reduced the need for hysterectomy but also because a laparoscopic hysterectomy, which is perfectly possible, is regarded by some gynaecologists as a rather more complicated version of a vaginal hysterectomy. Endoscopic operations on the fallopian tubes and the ovaries certainly have a place and are particularly valuable in preserving tubal function after the removal of an ectopic pregnancy for example. Laser ablation of endometriosis and the division of pelvic adhesions have been standard for many years and pelvic lymphadenectomy, which is a popular procedure in the United States, is an ideal operation for the endoscopist. For a detailed description of all these operations the reader is referred to a specialist text.
CONCLUSION
Endoscopic techniques are already widely used in other surgical specialties that we have not discussed. Arthroscopy has revolutionized operations on the knee in orthopaedics, for example, and nasal operations are much more accurate when viewed through an endoscope. Every branch of surgery will be influenced in due course and even now fine endoscopes are being made that can be inserted through a tiny burr hole into the subarachnoid space to examine the surface of the brain. It will not be long before neurosurgical operations are possible without the need for a craniotomy.
New operations and new ways of performing old operations are being developed with astonishing speed and endoscopic surgery is still in its infancy. Many future developments will depend on advances in technology but they will also depend on surgical skill. It is also of critical importance that proper evaluation of these developments takes place continually, not only in assessing the quality of the results of endoscopic surgery, but also the cost effectiveness of these procedures.
ГОУ ВПО «Смоленская государственная медицинская академия
Федерального агентства по здравоохранению и социальному развитию»
МЕТОДИЧЕСКИЕ УКАЗАНИЯ ДЛЯ СТУДЕНТОВ
ПО ДИСЦИПЛИНЕ хирургические болезни
HAEMORRHOIDS, ANAL FISSURE, RECTAL PROLAPSE
Составитель доц. Ю.И.Ломаченко
Методические указания утверждены на методическом совещании кафедры госпитальной хирургии (протокол № 2 от 6 октября 2008 г.)
Зав. кафедрой______________(проф. С.А.Касумьян)
2008 г.
UNIT: COLOPROCTOLOGY
Theme: SURGICAL ANATOMY AND PHYSIOLOGY OF THE RECTUM, ANUS AND ANAL CANAL
KEY QUESTIONS FOR HOMEWORK:
Anatomy of the rectum.
Definition of the anal canal.
Anal canal musculature.
Mechanism of defecation.
The mucous membrane.
The dentate line and the anal valves of Ball.
The crypts of Morgagni.
The anorectal ring.
Arterial supply, venous and lymphatic drainages.
Surgical physiology of the anal muscles and pelvic floor.
Anatomy of the rectum.
The rectum has an ill-defined ana-tomical beginning, the rectosigmoid junction lies opposite the sacral pro-montory. From here the rectum follows the curve of the sacrum, to end at the anorectal junction. At this point, the puborectalis muscle encircles the pos-terior and lateral aspects of the anorectal junction, creating the anorectal angle (normally 120°). The rectum has three lateral curvatures: the upper and lower are convex to the right, and the middle convex to the left (Houston's semicircular folds). That part of the rectum that lies below the middle valve has a much wider diameter and is known as the ampulla of the rectum. The adult rectum is approximately 18-20 cm in length. The rectum is separated by Denonvilliers' fascia – from the prostate, and Waldeyer's fascia from the coccyx and last two sacral vertebrae.
2. The anal canal commences at the level where the rectum passes through the pelvic diaphragm and ends at the anal verge (the external or distal boundary of the anal canal). The muscular junction between the rectum and anal canal can be felt with the finger as a thickened ridge - the anorectal «bundle» or «ring».
3. Anal canal musculature.
The internal sphincter is a thickened continuation of the circular muscle coat of the rectum. This involuntary muscle commences where the rectum passes through the pelvic diaphragm, and ends at the anal orifice, where its lower border can be felt. The internal anal sphincter is 2.5 cm long and 2 to 5 mm thick. When exposed during life, it is pearly white in colour, and its individual transversely placed fibres can be seen clearly. Spasm and contracture of this muscle play a major part in fissure and other anal affections.
The longitudinal muscle is a continuation of the longitudinal muscle coat of the rectum intermingled with fibres from the puborectalis. Its fibres fan out through the lowest part of the external sphincter, to be inserted into the true anal and perianal skin. The longitudinal muscle fibres that are attached to the epithelium provide pathways for»he spread of perianal infections, and mark out tight 'compartments' that are responsible for the intense pressure and pain that accompany many localised perianal lesions.
Beneath the anal skin lie the scanty fibres of the corrugator cutis ani muscle.
The external sphincter, formerly subdivided into a deep, superficial, and subcutaneous portion is now considered to be one muscle. Some of its fibres are attached posteriorly to the coccyx, while anteriorly they are inserted into the midperineal point in the male, whereas in the female they fuse with the sphincter vaginae. In life the external sphincter is pink in colour, and homogeneous. Unlike the pale internal sphincter muscle, which is involuntary, the red external sphincter is composed of voluntary (somatic) muscle.
Between the internal (involuntary) sphincter and the external (voluntary) sphincter muscle mass is found a potential space the intersphincteric plane. This plane is important as it contains the basal parts of 8-12 apocrine glands, which can cause infections, and it is also a route for the spread of pus. It can also be opened up by a surgeon to provide access for operations on the sphincter muscles.
The puborectalis plays a key role in maintaining the angle between the anal canal and rectum and, hence, is essential for the preservation of continence.There is a close association between the puborectalis portion of the levator ani and the external sphincter muscle.
Mechanism of defecation.
Muscles of defecation. The muscles that act on the rectal neck are the external and internal anal sphincters, puborectalis, levator ani and longitudinal muscle. The external and internal sphincters as well as the puborectalis are muscles of continence. Their role at stool is to contract in order to interrupt or terminate the act of defecation. However, the principal muscles of defecation are the levator ani and the longitudinal muscles. They act jointly to open the rectal neck at defecation.
Anatomical mechanism of defecation. As stools enter the rectum, reflex detrusor contraction and internal sphincter relaxation occur. The continuation of defecation depends on 2 factors: external sphincter relaxation and straining. If defecation is acceded to, the external sphincter is voluntarily relaxed. Straining is necessary to maintain defecation as it raises the intra-abdominal pressure. This serves a double purpose: it compresses the detrusor, which helps evacuation, and it stimulates levator contraction through the straining-levator reflex. Although the intra-abdominal pressure compresses the detrusor, the rectal neck is spared owing to its protected location below the levator plate. When the levator plate contracts, it moves from the cone to the flat position and is elevated and laterally retracted. This results in pulling on the hiatal ligament, which in turn pulls open the anorectal junction and partially opens the rectal angle. Simultaneously, the suspensory sling contracts and not only pulls up the base loop to unseal the anal orifice, but also partially opens the rectal neck. The longitudinal muscle joins the detrusor in contraction, which results in shortening and opening of rectal neck as well as in complete straightening of the rectal angle. This brings the rectal neck into alignment with the detrusor so that efficient fecal pumping occurs. The final result of the joint contraction of the detrusor, longitudinal muscle and levator ani is the opening of the rectal neck for the rectum to evacuate its contents.
Physiologic mechanism of defecation. The concerted functions of the anorectal musculature at defecation are initiated and harmonized by voluntary impulses and reflex actions. When the rectal detrusor is distended with fecal mass and the stretch receptors are stimulated, the recto-anal inhibitory reflex is initiated by which the rectal detrusor contracts and the internal sphincter relaxes. Detrusor contraction triggers 2 reflexes: recto-puborectalis reflex and the recto-levator reflex. These two reflexes act simultaneously, yet have opposite functions; on detrusor contraction, the recto-levator reflex effects a reflex levator contraction, which opens the rectal neck. At the same time, the reflex puborectalis contraction, actuated by the rectopuborectalis reflex, functions to close or keep closed the rectal neck as impulses reach the conscious level to probe the circumstances for defecation. If inopportune, the puborectalis continues voluntary contraction. Voluntary puborectalis contraction evokes 2 reflex actions: reflex levator relaxation through the levator-puborectalis reflex and reflex detrusor relaxation by means of the voluntary inhibition reflex. Meanwhile it aborts the recto-anal inhibitory reflex, which relaxes the internal sphincter. Hence voluntary puborectalis contraction, through the voluntary inhibition reflex, prevents internal sphincter relaxation, which results in reflex detrusor relaxation and waning of the urge to defecate. However, as soon as circumstances would allow defecation and the sensation of desire is felt, the puborectalis muscle relaxes voluntarily and the detrusor evacuates its contents. This demonstrates that the act of defecation is under voluntary control despite the presence of reflex actions sharing in the mechanism of defecation. Thus, although the recto-anal inhibitory and rectolevator reflexes function to open rectal neck, the recto-puborectalis and the levator-puborectalis reflexes keep the rectal neck closed until the decision for defecation has been taken. Straining at the start of defecation is a normal physiological process and as such is part of the mechanism of defecation. By elevating the intra-abdominal pressure, it triggers the straining-levator reflex, which effects levator contraction and the opening of the rectal neck for the spontaneous evacuation of the stools.
5. The mucous membrane. The pink columnar epithelium lining the rectum extends through the anorectal ring into the surgical anal canal. The mucosa of the upper anal canal is attached loosely to the underlying structures, and covers the internal rectal plexus. Passing downwards where it clothes the series of 8-12 longitudinal folds known as the columns of Morgagni, the mucous membrane becomes cubical, and red in colour; above the anal valves the mucous membrane becomes plum coloured. Just below the level of the anal valves there is an abrupt, albeit wavy, transition to squamous epithelium, which is parchment colour. This wavy junction constitutes the dentate line. The squamous epithelium lining the lower anal canal is thin and shiny, and is known as the anoderm. This squamous epithelium differs from the true skin in that it has no epidermal appendages, i.e. hair and sweat glands. The anoderm passes imperceptibly into the pigmented skin of the anus. At the dentate line the anoderm is attached very firmly indeed to deeper structures.
6. The dentate line is a most important landmark both morphologically and surgically. It represents the site of fusion of the proctodaeum and post-allantoic gut, and the position of the anal membrane, remnants of which may frequently be seen as anal papillas situated on the free margin of the anal valves. The dentate line separates:
above – • cubical epithelium
• autonomic nerves (insensitive)
• portal venous system
below – • from squamous epithelium
• from spinal nerves (very sensitive)
• from systemic venous system.
The anal valves of Ball are a series of transversely placed semilunar folds linking the columns of Morgagni. They lie along and actually constitute the waviness of the dentate line. They are functionless remnants of the fusion of the postallantoic gut with the proctodaeum.
7. The crypts of Morgagni (syn. anal crypts) are small pockets between the inferior extremities of the columns of Morgagni. Into several of these crypts, mostly those situated posteriorly, opens one anal gland by a narrow duct. This duct bifurcates, and the branches pass outward to enter the internal sphincter muscle, in 60 per cent of people. Issuing from this ampulla there are 3-6 tubular sub-branches that extend into the intermuscular connective tissue, where they end blindly. In some lower animals, these glands secrete an odoriferous substance during the rutting season; in humans, their function, if any, is obscure. Some of their cells have been shown to give a positive staining reaction for mucin, but as the lining epithelium is mainly cubical, the mucus-secreting propensity of the anal glands must be extremely small. Infection of an anal gland can give rise to an abscess, and in the opinion of a number of surgeons, infection of an anal gland is the most common cause of anorectal abscesses and fistulas.
8. The anorectal ring marks the junction between the rectum and the anal canal. It is formed by the joining of the puborectalis muscle, the deep external sphincter, conjoined longitudinal muscle, and the highest part of the internal sphincter. The anorectal ring can be clearly felt digitally, especially on its posterior and lateral aspects. Division of the anorectal ring results in permanent incontinence of faeces. The position and length of the anal canal, as well as the angle of the anorectal junction, depends to a major extent on the integrity and strength of the puborectalis muscle sling.
9. Arterial supply. The anal canal is supplied by branches from the superior, middle and inferior haemorrhoidal arteries. The most important is the superior haemorrhoidal, whose left branch supplies the left half of the canal by a single terminal branch, while its right has two terminal branches. All the arteries contribute to a rich submucous and intramural plexus, so that interruption of the arterial supply from above by division of the superior and middle rectal arteries does not deprive the anus of its blood supply.
Venous drainage. The anal veins are distributed in similar fashion to the arterial supply. The superior and middle haemorrhoidal veins drain via the inferior mesenteric vein into the portal system, having become the superior rectal vein en route. The superior haemorrhoidal vein drains the upper half of the anal canal. The inferior haemorrhoidal veins drain the lower half of the anal canal and the subcutaneous perianal plexus of veins: they eventually join the external iliac vein on each side.
Lymphatic drainage. Lymph from the upper half of the anal canal flows upwards to drain into the postrectal lymph nodes and from there goes to the para-aortic nodes via the inferior mesenteric chain. Lymph from the lower half of the anal canal drains on each side first into the superficial and then into the deep inguinal group of lymph glands. However, if the normal flow is blocked, e.g. by tumour, the lymph can be diverted into the alternative route.
10. Surgical physiology of the anal muscles and pelvic floor.
The function of the anal canal and pelvic floor muscles is to not only contain the contents of the rectum, but to allow effortless, unimpeded voiding at defaecation. Interference with the integrity of the anatomy or priysiology of the muscles of the anus and pelvic floor can lead to the extremes of intractable constipation or incontinence. If the muscles of the pelvic floor become too floppy, the entire anorectal mechanism can drop down («perineal descent»), or alternatively can gape open, so allowing intussusception and prolapse of the rectum.
If the puborectalis and anorectal ring of muscles fail to relax appropriately (so-called «inappropriate function» or anismus) to allow the rectum to empty at defaecation, obstructed defaecation ensues: this can usually be overcome by excessive voluntary straining efforts, but frequently ends in intractable constipation. Excessive straining can cause both partial and complete rectal prolapse.
UNIT: COLOPROCTOLOGY
Theme: CLINICAL FEATURES OF RECTAL DISEASES AND EXAMINATION OF THE RECTUM AND ANUS
KEY QUESTIONS FOR HOMEWORK:
Symptoms of rectal diseases.
Conditions examination and inspection.
Digital examination with the index finger.
Anoscopy (proctoscopy).
Rectosigmoidoscopy.
Flexible sigmoidoscopy.
Colonoscopy or barium enema.
Special investigations.
1. Symptoms.
Rectal diseases are common, serious and can occur at any age. The symptoms of many of them overlap. In general, the inflammations affect younger age groups, while the tumours occur in the middle-aged and elderly. But no age is exempt from any of the diseases, however young: ulcerative colitis has been reported in the newborn, and rectal cancer is not rare in young people. The common symptoms of rectal disease are:
Bleeding. Bright red blood on the toilet paper on wiping the anus is a common symptom of haemorrhoids. There are many other causes. This demands at least digital examination at any age.
Altered bowel habit. Early morning stool frequency («spurious diarrhea») is a symptom of rectal carcinoma, while bloodstained frequent loose stools characterise the inflammatory diseases.
Discharge. Mucus and pus are associated with rectal pathology.
Tenesmus. Often described by the patient as «I feel I want to go but nothing happens»; this is normally an ominous symptom of rectal cancer.
Prolapse. This usually indicates either mucosal (partial) or full-thickness (complete) rectal wall descent.
Pruritus. This may be secondary to a rectal discharge.
Loss of weight. This usually indicates serious or advanced disease, e.g. hepatic metastases.
Signs of rectal diseases can be elicited by systematic examination. The rectum is accessible via the anal orifice.
2. Examination of the anus requires careful attention to circumstances. The examining couch should be of sufficient height to allow easy inspection and access for any necessary manoeuvres. A good light is mandatory. The Sims (left lateral position, with the buttocks projecting slightly beyond the edge of the table) or the prone jackknife position or the lithotomy position are satisfactory: the latter is less convenient for an elderly patient and can cause social embarassment to young women. A protective glove should be worn. The patient should be relaxed and able to co-operate. A few quiet words from the doctor can prevent many loud ones from the patient.
Inspection. With the buttocks opened, the anus is inspected. Note is made of any lesions, e.g. inflammatory skin changes, haemorrhoids, fissure («sentinel pile»), or fistula. A patulous anus may indicate incontinence and possibly prolapse. The patient is asked to strain down before inspection is concluded. Visual examination of the anus precedes rectal examination to exclude the presence of anal disease, e.g. fissure, haemorrhoids, fistula, etc.
3. Digital examination with the index finger. The index finger used with gentleness and precision remains the most valuable test for rectal disease. A good lubricant is necessary - neither too little nor too much. Any secretions should be sampled before applying lubricant to the anal verge.
Extreme gentleness should be the rule so that pain is not caused. A careful and systematic digital examination with a well-lubricated index finger gradually inserted into the anal canal helps the examiner to appreciate any mass, induration, or stricturing as well as to assess the resting tone and strength of the squeeze pressure of the anal sphincter. Painful spasm of the anal sphincters is confirmation of a hidden fissure if the history is suggestive.
Tumors in the lower and middle thirds of the rectum can be felt and assessed; by asking the patient to strain, even some tumours in the upper third can be 'tipped' with the finger. Before withdrawing the finger, the patient is asked again to strain down, and a note is made regarding the prostate in a male patient and the cervix, uterus and pouch of Douglas in a female. After withdrawal, the finger is examined for mucus, pus, blood and abnormal faecal material. Digital findings can be recorded as intraluminal (e.g. blood, pus etc.), intramural (e.g. tumours, granular areas, strictures, etc.) or extramural (e.g. enlarged prostate, uterine fibroids, etc.).
4. Anoscopy (proctoscopy). This examination is of great importance. Either the Sims position with the buttocks elevated on a small cushion, or the knee-elbow position may be used. The lower third of the rectum, the anorectal junction and the anal canal can be inspected up to 10 cm as the instrument is withdrawn slowly. The patient should also be asked to strain during withdrawal as by so doing an internal intusus-sception may be made visible. Minor procedures can be carried out through this instrument, e.g. treatment of haemorrhoids by injection or banding and biopsy. Biopsy can be performed of any suspicious areas.
5. Rectosigmoidoscopy. The rectosigmoidoscope is a rigid, stainless-steel instrument of variable diameter and normally 25 cm in length. The rigid rectosigmoidoscope allows inspection of only the lower 20-25 cm of the bowel. The rectum must be empty for proper inspection with a rectosigmoidoscope. Gentleness and skill are required for its use, and perforations can occur if care is not exercised. Early signs of mucosal inflammation include the loss of the vascular pattern with erythema, granularity, friability, and even ulcerations.
6. Flexible sigmoidoscopy. Although this is strictly an examination of the rectum and lower sigmoid colon, it should be carried out even when an anal lesion has been confirmed. Rectal pathology, e.g. colitis or carcinoma, is frequently the cause of an anal lesion, e.g. fissure or haemorrhoids. Not infrequently, rectal pathology is found that is independent of the anal lesion and which requires treatment. The 'flexiscope' can be used to supplement or replace rigid rectosigmoidoscopy. It requires special skill and experience, and the lower bowel should be cleaned out with preliminary enemas. In addition to the rectum, the whole sigmoid colon is within visual reach of this instrument. A 70 cm flexible sigmoidoscope allows much more bowel to be visualized. The instrument is expensive and requires careful maintenance. Seventy per cent of colonic neoplasms occur within the range of the flexible sigmoidoscope.
7. Colonoscopy or barium enema should be added if the disease is unimpressive, the history is somewhat uncharacteristic, or the patient is older than 40 years of age or has risk factors for colon cancer, such as a family history.
8. Special investigations. The length, resting tone and the power to relax and contract the anal sphincter muscles can be assessed by manometry and electromyography: these studies can be combined with delineation of rectal sensibility and function by balloon distension and radiology («defaecatory proctography») and the abnormalities identified. In addition to the intrinsic defects, mechanical deviations can also be mapped: the level and angle of the anorectal junction can be established by clinical observation, and by an appliance («perineometer») . Furthermore, it is possible to take radiographs of the acts of straining and evacuation while simultaneously recording electro-myographs of the sphincter muscles and intrarectal pressure. This integrated dynamic proctography, together with the other techniques of investigation, provides information which enables many patients with incontinence and constipation to be treated effectively.
UNIT: COLOPROCTOLOGY
Theme: HAEMORRHOIDS
KEY QUESTIONS FOR HOMEWORK:
Haemorrhoids’ definition and aetiology.
Surgical anatomy.
Clinical symptoms of haemorrhoids and its complications.
Investigation.
Differential diagnosis.
Treatment.
Haemorrhoids (Greek - haima = blood, rhoos = flowing) syn. piles (Latin - pila = a ball).
Within the normal anal canal exist specialized, highly vascularized "cushions" forming discrete masses of thick submucosa containing blood vessels, smooth muscle, and elastic and connective tissue. There are 3 main cushions located in the left lateral (3 o'clock), right posterior (7 o'clock), and right anterior (11 o'clock) sectors (quadrants) of the canal (anal circumference) to aid in anal continence. Minor tufts can be found between the cushions. The anal cushions are present in embryonic life and are necessary for full continence.
The term hemorrhoids should be restricted to clinical situations in which these "cushions" are abnormal and cause symptoms. The causes of haemorrhoids may be no more than the downward sliding of anal cushions associated with gravity, straining, and irregular bowel habits. The condition is so frequently seen in members of the same family that there must be a predisposing factor, such as a congenital weakness of the vein walls or an abnormally large arterial supply to the rectal plexus. Varicose veins of the legs and haemorrhoids often occur concurrently.
Haemorrhoids may be symptomatic of some other condition, and this important fact must be remembered. The great majority of haemorrhoids are not symptomatic. Symptomatic haemorrhoids may appear:
• in carcinoma of rectum. This, by compressing or causing thrombosis of the superior rectal vein, gives rise to haemorrhoids sufficiently often to warrant examination of the rectum and the rectosigmoid junction for a neoplasm in every case of haemorrhoids;
• during pregnancy. Pregnancy piles are due to compression of the superior rectal veins by the pregnant uterus and the relaxing effect of progesterone on the smooth muscle in the walls of the veins, plus an increased pelvic circulating volume;
• from straining at micturition consequent upon a stricture of the urethra - or an enlarged prostate;
• from chronic constipation.
Haemorrhoids may be external or internal, i.e. external or internal to the anal orifice. The external variety are covered by skin, while the internal variety lie beneath the anal mucous membrane. When the two varieties are associated, they are known as interoexternal haemorrhoids.
2. Surgical anatomy.
Hemorrhoids are categorized into internal and external hemorrhoids. These categories are anatomically separated by the dentate (pectinate) line. Because of the communication between the internal and external plexuses, if a dilatation of the internal venous plexus becomes, the external venous plexus is liable to become enlarged also. External hemorrhoids are hemorrhoids covered by squamous epithelium, whereas internal hemorrhoids are lined with columnar epithelium. Similarly, external hemorrhoids are innervated by cutaneous nerves that supply the perianal area. These nerves include the pudendal nerve and sacral plexus. Internal hemorrhoids are not supplied by somatic sensory nerves and therefore cannot cause pain. At the level of the dentate line, internal hemorrhoids are anchored to the underlying muscle by the mucosal suspensory ligament. Internal haemorrhoids are frequently arranged in three groups at 3, 7, and 11 o'clock with the patient in the lithotomy position (in which patients used to be put for the classic operation of «cutting» for bladder stone via the urethral or the perineal route). Each principal haemorrhoid can be divided into three parts:
• The pedicle is situated at the anorectal ring. As seen through a proctoscope, it is covered with pale pink mucosa. Occasionally, a pulsating artery can be felt in this situation. Entering the pedicle of an internal haemorrhoid may be a branch of the superior rectal artery.
• The internal haemorrhoid, which commences just below the anorectal ring. It is bright red or purple, and covered by mucous membrane. It is of variable size.
• An external associated haemorrhoid lies between the dentate line and the anal margin. It is covered by skin, through which blue veins can be seen, unless fibrosis has occurred. This associated haemorrhoid is present only in well-established cases.
Evidence indicates that hemorrhoidal bleeding is arterial and not venous. This evidence is supported by the bright red color and arterial pH of the blood.
Clinical symptoms of haemorrhoids and its complications.
INTERNAL HAEMORRHOIDS.
Bleeding, as the name haemorrhoid implies, is the principal and earliest symptom. The capillaries of the lamina propria are only protected by a single layer of epithelial cells, and little trauma is required to breach them. Since it is the more lax-textured, upper part of the anal cushion which mainly prolapses, dragging the mucosa to the outside, trauma due to wiping or contact with clothes often occurs. Repeated trauma produces a chronic inflammatory response, making the damaged mucosa a brighter red and granular, and so more friable and likely to bleed. Internal hemorrhoids most commonly cause painless bleeding with bowel movements. Haemorrhoids that bleed but do not prolapse outside the anal canal are called first-degree (grad I) haemorrhoids. Patients may notice red blood on their toilet paper on wiping the anus. It is a common symptom of hemorrhoids.
Anaemia can be caused very rarely by persistent profuse bleeding from haemorrhoids.
Prolapse is a much later symptom. In the beginning the protrusion is slight and occurs only at stool, and reduction is spontaneous. As time goes on the haemorrhoids do not reduce themselves, but have to be replaced digitally by the patient. Haemorrhoids that prolapse on defecation but return are called second-degree (grad II) haemorrhoids (prolapse with Valsalva and spontaneous reduction). Haemorrhoids that prolapse on defaecation but need to be replaced manually and then stay reduced are called third-degree (grad III) haemorrhoids (prolapse with Valsalva requires manual reduction). Still later, prolapse occurs during the day, apart from defecation, often when patients are tired or exert themselves. Haemorrhoids that are permanently prolapsed are called fourth-degree (grad IV) haemorrhoids (chronically prolapse manual reduction ineffective). By now, the haemorrhoids have become a source of great discomfort and cause a feeling of heaviness in the rectum but are not usually acutely painful. Internal hemorrhoids can deposit mucus onto the perianal tissue with prolapse. A mucoid discharge is a frequent accompaniment of prolapsed haemorrhoids. This mucus with microscopic stool contents can cause a localized dermatitis, which is called pruritus ani.
Pain is absent unless complications supervene. Internal hemorrhoids can cause perianal pain by prolapsing and causing spasm of the sphincter complex around the hemorrhoids. Internal hemorrhoids can also cause acute pain when incarcerated and strangulated. Again, the pain is related to the sphincter complex spasm. Strangulation with necrosis may cause more deep discomfort. When these catastrophic events occur, the sphincter spasm often causes concomitant external thrombosis. External thrombosis causes acute cutaneous pain. Thus internal haemorrhoids are classified as:
First-degree (grad I) |
Only bleeding indicates presence, no prolapse |
Second-degree (grad II) |
Bleeding, pruritus, mild discomfort, prolapse usually with defecation, spontaneous reduction |
Third-degree (grad III) |
Bleeding, pruritus, discharge pain, prolapse requiring manual replacement |
Fourth-degree (grad IV) |
Bleeding, pain, incontinence, permanent prolapse, reduction impossible |
EXTERNAL HAEMORRHOIDS.
Unlike internal haemorrhoids, external haemorrhoids consist of a conglomerate group of distinct clinical entities. External hemorrhoids can cause trouble with hygiene. The excess skin left after an acute thrombosis (skin tags) is actually accountable for these problems. A thrombosed external haemorrhoid is commonly termed a perianal haematoma. It is a small clot occurring in the perianal subcutaneous connective tissue. The condition appears suddenly and is very painful, and on examination a tense, tender swelling. Untreated it may resolve, suppurate, fibrose, and give rise to a cutaneous tag, or burst and extrude the clot, or continue bleeding. In the majority of cases resolution or fibrosis occurs. Indeed, this condition has been called «a 5-day, painful, self-curing lesion» (Milligan).
Complications of haemorrhoids.
Profuse haemorrhage is not rare.
«Acute attack of piles» (strangulation of internal hemorrhoids – one or more of the internal haemorrhoids prolapse and become gripped by the external sphincter; thrombosis of hemorrhoids – unless the internal haemorrhoids can be reduced within an hour or two, strangulation is followed by thrombosis; oedema and inflamation of the anal skin – сonsiderable oedema of the anal margin accompanies thrombosis; ulceration of hemorrhoids – superficial ulceration of the exposed mucous membrane often accompanies strangulation with thrombosis; gangrene of internal hemorrhoids – it occurs when strangulation is sufficiently tight to constrict the arterial supply of the haemorrhoid, massive gangrene extends to the mucous membrane within the anal canal and rectum and can be the cause of spreading anaerobic infection and portal pyaemia; anopectal suppuration – a perianal or submucous abscess occur as a result of infection of a thrombosed haemorrhoid).
Fibrosis. After thrombosis, internal haemorrhoids sometimes become converted into fibrous tissue. The fibrosed haemorrhoid is at first sessile, but by repeated traction during prolapse at defaecation, it becomes pedunculated and constitutes a fibrous polyp that is readily distinguished by its white colour from an adenoma, which is bright red. Fibrosis following transient strangulation commonly occurs in the subcutaneous part of a primary haemorrhoid. Fibrosis in an external haemorrhoid favours prolapse of an associated internal haemorrhoid.
Pylephlebitis (syn. portal pyaemia) is surprisingly infrequent. It can occur when patients with strangulated haemorrhoids are subjected to ill-advised surgery.
4. Investigation.
On inspection there may be no evidence of internal haemorrhoids. In more advanced cases, redundant folds or tags of skin can be seen in the position of one or more of the three primary haemorrhoids. When the patient strains, internal haemorrhoids may come into view transiently or, if they are of the third degree, they are, and remain, prolapsed.
Digital examination. Internal haemorrhoids cannot be felt unless they are thrombosed.
Anoscopy (proctoscopy). A proctoscope is passed to its fullest extent and the obturator is removed. The instrument is then slowly withdrawn. Just below the anorectal ring internal haemorrhoids, if present, will bulge into the lumen of the proctoscope.
Sigmoidoscopy should be done as a precaution in every case.
Laboratory evaluation is not typically required.
5. Differential diagnosis.
Differential diagnosis of haemorrhoids includes anal tags, fibrous anal polyp, anal fissure, dermatitis, perianal haematoma, rectal prolapse, and rectal tumor.
6. Treatment
Nonoperative treatment
Nonoperative treatment may be recommended when the haemorrhoids are called first-degree (grad I) and second-degree (grad II) haemorrhoids or are a symptom of some other condition or disease except, of course, when a carcinoma is present.
Dietary management consisting of adequate fluid and fiber intake is the primary noninvasive treatment of symptomatic hemorrhoids. The bowels are regulated by hydrophyllic colloids. A high-fiber diet includes more than 25 grams of fiber per day. Psyllium seed and methylcellulose are the most commonly used fiber supplements.
Antidiarrheal agents are sometimes required in patients with symptoms and loose stools. Toilet retraining involves reminding patients that the lavatory is not the library. Patients should sit on the toilet only long enough to evacuate the lower intestines. Persistent straining or prolonged sitting can lead to engorged hemorrhoids.
Various proprietary creams can be inserted into the rectum from a collapsible tube fitted with a nozzle, at night and before defaecation. Topical hydrocortisone can sometimes ease internal haemorrhoidal bleeding.
Suppositories are also useful.
Nonoperative treatment is ineffective for hemorrhoids with significant prolapse (grades III-IV) and consideration should be given to more aggressive treatment modalities. Most patients with refractory to nonoperative treatment (grades I-II) are candidates for other procedures.
Active treatment (office-based procedures).
Office treatment of haemorrhoids includes several procedures, which all attempt to decrease vascularity, decrease haemorrhoidal volume, and increase fixation of the fibrovascular cushion to the rectal wall. This leads to improvement in the specific symptoms of prolapse and rectal bleeding.
Banding treatment (rubber-band ligation). Haemorrhoid banding is usually the most effective option according to clinical practice guidelines of the American Society of Colon and Rectal Surgeons. It is associated with a lower recurrence rate but more overall pain than sclerotherapy or infrared coagulation. For second-degree haemorrhoids which are too large for successful handling by injections, treatment is available by slipping tight elastic bands on to the base of the pedicle of each haemorrhoid with a special instrument.The bands cause ischaemic necrosis of the piles, which slough off within a few days. Not more than two haemorrhoids should be banded at each session and 3 weeks at least should elapse between each treatment.
Injection treatment (sclerotherapy injection). This is ideal for first-degree internal haemorrhoids which bleed. Early second-degree haemorrhoids are often cured by this method but a proportion relapse. Sclerotherapy involves injecting a sclerosant into the apex of the haemorrhoid and is effective in 75 to 89 percent of patients with for grades I, II, and III hemorrhoids.
Cryosurgery (syn. carbon dioxide freezing). The application of liquid nitrogen has been evaluated in some centres. The extreme cold (-196°C) of the application causes cold necrosis of the piles which subsequently separate and drop off. The technique often tends to cause troublesome mucus discharge, which has limited its use.
Infrared photocoagulation. The application of infrared coagulation by a specially designed instrument has been advocated for the treatment of haemorrhoids that do not prolapse. This is said to be an effective and painless method of treatment. Infrared coagulation involves direct application of infrared waves resulting in protein necrosis. It is most applicable to grades I and II haemorrhoids but is associated with high rates of recurrence when substantial prolapse is present.
Bicap coagulation (bipolar current or bipolar diathermy). The application of bipolar current has proven useful in the treatment of grades I to III haemorrhoids. Bicap coagulation lasts only several seconds and are associated with a minor complication rate of 10 percent and recurrence rates between 25 and 35 percent.
Operation (surgical resection).
1) Surgical haemorrhoidectomy is the most effective treatment for haemorrhoidal disease. However, it also was associated with the highest complication rate and the most postoperative disability. As such, individual patient factors and preferences need to be carefully weighed and considered. Surgery is the replacement of one lesion by another; the aim is to ensure that the second is preferable to the first. Surgical options include open or closed haemorrhoidectomy performed with surgical scalpel, diathermy, laser, or ultrasonic scalpel.
Indications for haemorrhoidectomy:
• third-degree (grad III) and fourth-degree (grad IV) haemorrhoids;
• failure of nonoperative treatments of second-degree (grad II) haemorrhoids;
• fibrosed haemorrhoids;
• interoexternal haemorrhoids when the external haemorrhoid is well defined.
Haemorrhoidectomy should be reserved according to clinical practice guidelines of the American Society of Colon and Rectal Surgeons: for patients refractory to office procedures, unable to tolerate office procedures, patients with large external haemorrhoids, or patients with combined internal and external haemorrhoids with significant prolapse (grades III-IV).
Haemorrhoidectomy can be performed using an open or closed technique. The open technique is most commonly used in the UK, and is known as the Milligan Morgan’s operation - named after the surgeons who described it. The closed technique is the popular technique in the USA and Russia. Both involve ligation and excision of the haemorrhoid, but in the open technique the anal mucosa and skin are left open to heal by secondary intention, and in the closed technique, the wound is sutured.
2) Stapled haemorrhoidectopexy (haemorrhoidectomy) is a new alternative available for individuals with significant haemorrhoidal prolapse. It involves a mucosal and submucosal, circular resection of the haemorrhoidal columns at their apex. In addition, the blood supply is interrupted and haemorrhoids are “fixed" to the distal rectal muscular wall. This is all accomplished by a single firing of a modified, circular anastomotic stapler. Exceptionally rare but potentially devastating complications include anovaginal fistula, substantial hemorrhage, fistula, retroperitoneal sepsis, and rectal perforation. The stapling procedure is not effective for treating large external hemorrhoids.
3) Surgical submucosal haemorrhoidectomy (Parks’ operation). The mucosa is incised, the haemorrhoid is excised submucosal, the wound is sutured.
Postoperative complications of haemorrhoidectomy may be early or late.
1) Early postoperative complications.
Retention of urine is not unusual after haemorrhoidectomy in male patients. Before resorting to catheterisation, the patient should be reassured, given an analgesic, allowed to stand at the side of the bed in privacy or be assisted to a hot bath into which he may be able to void urine.
The haemorrhage may be mainly or entirely concealed, but will become evident on examining the rectum. If the bleeding persists, the patient must be taken to the operating theatre and the bleeding-point secured by diathermy or under-running with a ligature on a needle. Should a definite bleeding-point not be found, suspected areas are under-run in this way.
2) Late postoperative complications.
Secondary haemorrhage is uncommon; when it occurs, it does so about the 7th or 8th day after operation. If the haemorrhage is severe, an anaesthetic should be given and a catgut stitch inserted to occlude the bleeding vessel.
Anal stricture. This must be prevented at all costs. A rectal examination at the 10th day will indicate if stricturing is to be expected. It may then be necessary to give a general anaesthetic and dilate the anus. After that, daily use of the dilator should give a satisfactory result.
Anal fissure and submucous abscess may also occur.
TREATMENT OF COMPLICATIONS
Strangulation, thrombosis and gangrene. Treatment options include observation or excision. Excision, within 48 to 72 hours of onset of symptoms will result in the most rapid relief from symptoms. It was formerly believed that surgery would promote pylephlebitis. If adequate antibiotic cover is given from the start, this is not found to be so and immediate surgery can be justified in many patients. Besides adequate pain relief (patient analgesia), bed rest with frequent, ice or sitz baths to the perineum, avoidance of constipation may result in more rapid symptom relief than will surgical excision or usually cause the pile mass to shrink considerably in 3 or 4 days when standard ligation and excision of the piles can be carried out. Some surgeons consider that the operation at this stage increases the risk of postoperative stenosis and delay surgery for a month or so. They then review the situation and only carry out haemorrhoidectomy if necessary. In spite of the low risk of pylephlebitis, caution should dictate a «noninterventionist» policy whenever this is practical. An anal dilation technique can be used as a useful alternative treatment to surgery for painful «strangulated» haemorrhoids.
Thrombosis of the external haemorrhoid.
Provided it is seen within 36 hours of the onset, a perianal haematoma is best treated as an emergency. Under local anaesthesia the haemorrhoid is bisected and the two halves are excised together with 1-25 cm of adjacent skin. This leaves a pear-shaped wound which is allowed to granulate.
Severe haemorrhage. After blood replacement is adequate, ligation and excision of the piles may be required.
UNIT: COLOPROCTOLOGY
Theme: ANAL FISSURE
KEY QUESTIONS FOR HOMEWORK:
Definition, location and aetiology.
Classification and characteristic of the anal fissures.
Clinical features.
Investigation.
Differential diagnosis.
Treatment.
1. Definition. An anal fissure (syn. fissure in ano) is a longitudinal tear or elongated ulcer in the vertical axis of the squamous lining of the anal canal between the anal verge and the dentate line. An appearance of the anal fissure frequently is caused by the passage of a constipated stool, although, in a small proportion of patients, it may follow an episode of diarrhoea. The poor vascularization of the anal canal together with the frailty of the tissue, create favorable conditions to the appearing and continuity of the lesion.
Location. In 75 to 94 per cent of cases the fissure is situated at the posterior anal margin: anterior fissures are more commonly encountered in women. Simultaneous anterior and posterior fissures appear more rarely.
Aetiology. The trauma on the anal canal during defecation is accepted as the determining initial cause of the disease development. The cause of anal fissure, and particularly the reason why the midline posteriorly is so frequently affected, is not completely understood. A probable explanation is as follows: the posterior wall of the rectum curves forwards from the hollow of the sacrum to join the anal canal, which then turns sharply backwards. During defecation the pressure of a hard faecal mass is mainly on the posterior anal tissues, in which event the overlying epithelium is greatly stretched and, being relatively unsupported by muscle, is placed in a vulnerable position. An anterior anal fissure is much more common in women, particularly in those who have borne children. This can be explained by the lack of support of the anal mucous membrane by a damaged pelvic floor and an attenuated perineal body. Some causes of anal fissure are certain:
an incorrectly performed operation for hemorrhoids in which too much skin is removed. This results in anal stenosis and tearing of the scar when a hard motion is passed. The lack of elasticity of the anal canal because of pathological or postoperative fibrosis predisposes to trauma;
inflammatory bowel disease - particularly Crohn's disease;
sexually transmitted diseases;
anal fissures frequently develop in patients who used laxatives in excess in the postoperative period.
2. Pathology. An anal fissure is either acute or chronic. The upper internal end of the fissure stops at the dentate line. Because the fissure occurs in the stratified sensitive epithelium of the lower half of the anal canal, pain is the most prominent symptom.
Acute anal fissures are superficial, but may deepen to expose the underlying internal sphincter. It is a deep tear through the skin of the anal margin extending into the anal canal. There is little inflammatory induration or oedema of its edges. There is accompanying spasm of the anal sphincter muscle.
Chronic anal fissure is characterised by inflamed indurated margins (induration of the edge of the fissure), and a base consisting of either scar tissue or the lower border of the internal sphincter muscle. The ulcer is canoe-shaped, and at the inferior extremity frequently there is a tag of skin, usually oedematous. This tag is known picturesquely as a sentinel pile – «sentinel» because it guards the fissure. There may be spasm of the involuntary musculature of the internal sphincter. The reflex relaxation of the internal sphincter that normally follows defecation is lost in patients with anal fissure; instead contraction of the internal sphincter occurs. In long-standing cases, this muscle becomes organically contracted by infiltration of fibrous tissue. Infection is common and may be severe, ending in abscess formation. A vicious cycle ensues in which subepithelial inflammation causes spasm of the internal sphincter, inhibiting free drainage of the infected fissure and permitting continued inflammation, resulting in a small, chronic, inadequately drained abscess. A cutaneous fistula may follow.
Chronic fissure in ano may have a specific cause – often a granulomatous infection, e.g. Crohn's disease or syphilis. Biopsy examination is advisable of any tissue removed at operation for a chronic fissure. Specific fissures of this type are often less painful than the appearances of the lesion would suggest.
3. Clinical features. The condition is more common in women, and generally occurs during the meridian of life. It is uncommon in the aged, because of muscular atony; on the other hand, anal fissure is not rare in children, is sometimes encountered during infancy, and may cause acquired megacolon. Anal fissures cause painful defecation and minor rectal bleeding.
• Pain is the symptom - sharp, agonising pain starting during defecation, often overwhelming in intensity and lasting an hour or more. As a rule, it ceases suddenly, and the sufferer is comfortable until the next action of the bowel. Periods of remission occur for days or weeks. The patient tends to become constipated rather than go through the agony of defecation. The pain reflects on all the aspects of every day patients’ life, which starts to be organized according to the pain cycle. There may be difficulty to urinate in consequence of the pain reflex mechanism. At the end of urination, the contraction of the perineal muscles increases the fissure pain so that the patients starts to fear urinating, too. The urinary retention can occur especially in men. In the same way, the pain affects sexual activity, which starts to be avoided.
• Bleeding - this is usually slight and consists of bright streaks on the stools or the paper. The sphincter contraction causes ischemia of the anal canal, mainly in the area of the posterior commisure, where vascularization is very poor, consequently making healing difficult. Ischemia explains why anal bleeding in patients with anal fissure is not significant.
• Discharge. A slight discharge accompanies fully established cases.
4. Investigation.
The anamnesis of the patient with anal fissure is so characteristic that by itself gives us the diagnosis. If it is admitted that the process starts by the trauma of the anal canal, it is important to determine if it was consequent to evacuation or self-inflicted, and for this reason needs a more specific diagnostic approach, as to investigate sexually transmitted diseases. The disease causes constipation due to pain, so that the patient with fissure and diarrhea must be investigated as to the possibility of an inflammatory bowel disease, which can be a hidden cause of the fissure.
Local examination may be totally impracticable because of the fear of pain and anal contraction. In cases of some standing, a sentinel skin tag can usually be displayed. This, together with a typical history and a tightly closed, puckered anus, is almost pathognomonic of the condition. The lower end of the anal fissure can often be seen in the anal margin on inspection by gently parting the margins of the anus.
Because of the intense pain it causes, digital examination of the anal canal should not be attempted at this stage unless the fissure cannot be seen, or it seems imperative to exclude major intrarectal pathology. In these circumstances, the local application of a surface anaesthetic such as 5 per cent Xylocaine on a pledget of cotton wool, left in place for about 5 minutes, will enable the necessary examination to be made. In early cases, the edges of the fissure are impalpable; in fully established cases, a characteristic crater which feels like a vertical buttonhole can be palpated. The diagnosis must be established beyond doubt, for which a general anaesthetic may be required.
5. Differential diagnosis.
Carcinoma of the anus in its very early stages easily simulates a fissure. If real doubt exists, the lesion must be excised under general anaesthesia and submitted to histological examination.
Multiple fissures in the perianal skin are commonly seen as a complication of skin diseases, scratching and inflammatory bowel disease. Also homosexual practices (sodomy, fisting and the use of anorectal sex toys) and anorectal sexually transmitted disease can cause multiple fissures in both sexes.
Anal chancre is becoming more common and may present as a painful rather than a painless ulcer. The serous discharge contains spirochaetes. A glass pipette is used to aspirate a few drops which are placed on a slide for examination by dark-ground illumination. Lubricating gel from the fingerstall may prevent adequate aspiration of serum from a chancre. All patients with anal sexually transmitted disease, and admitted homosexuals, should be tested for a positive serological response to HIV as they may have AIDS.
Tuberculous ulcer has an undermined edge.
Proctalgia fugax causes severe episodic pain.
6. Treatment.
The pain of an anal fissure is so great that usually the patient demands relief, and consequently many patients with an acute fissure present early. The object of all treatment for this condition is to obtain complete relaxation of the internal sphincter. Provided the complications are dealt with, the fissure will slowly heal as soon as all spasm has disappeared.
Conservative treatment. Conservative therapy is safe, has few side effects, and should usually be the first step in therapy. In cases where the fissure is acute and superficial and where the inflammation is minimal, simple conservative measures will usually give relief.
Treatment is by application of a local anaesthetic gel. Adjunctive measures such as topical anesthetics cause no harm or decrease in healing rate, and may be used for patient comfort. Local anesthesia, with delayed action or not, with ointments or suppositories, present very irregular results, perhaps because of the difficulty the patient has when using them, due to pain.
Healing rates of 80 per cent have been reported following 3 weeks' treatment with Proctosedyl ointment (cinchocaine anaesthetic 0.5 per cent and hydrocortisone 0.5 per cent).
Anal fissures may be appropriately treated with topical nitrates because they can relieve pain; however, nitrates are only marginally associated with a healing rate superior to placebo. The most common used bases are the nitroglycerine and isosorbide. Use of topical nitroglycerin significantly decreases pain during the therapy period. The principal side effect is headache, which is dose-related. Patients who do not respond to topical therapy should be referred for surgical treatment.
Anal fissures may be appropriately treated with topical calcium channel blockers, which seem to have a lower incidence of adverse effects than nitrates. There is insufficient data to conclude whether they are superior to placebo in healing fissures. Topical calcium channel blockers have been associated with healing in 65 to 95 percent of chronic fissures according to data of some researches. The principal side effects are headache, seen in up to 25 percent of patients, flushing, and less commonly symptomatic hypotension.
Botulinum toxin injections may be used for anal fissures that fail to respond to conservative measures and have been associated with a healing rate superior to placebo. There is inadequate consensus on dosage, precise site of administration, number of injections or efficacy. Injection of botulinum toxin into the internal sphincter produces a temporary «chemical sphincterotomy».
Dilatation is not required.
Laxatives are prescribed to ensure that the motions are soft, but the stools should not be made watery.
Increased fluid and fiber ingestion, the use of sitz baths, and if necessary, the use of stool softeners often diminish bleeding and pain, and should be instituted as a first step in virtually all patients with a fissure.
Tranquilizers are also recommended because patients with fissure are always extremely anxious due to the symptoms.
Hygienic good habits contribute to avoid some recurrences.
These measures result in healing of up to 50 percent of symptomatic fissures.
Operative measures. If the fissure is chronic with fibrosis, a skin tag, or a mucous polyp, then surgical measures are advisable. General anaesthesia is best, though some surgeons use a local anaesthetic in the form of Xylocaine or lignocaine introduced into the ischiorectal fossa on each side, in order to anaesthetise the nerves passing towards the rectum. In other situations, a caudal anaesthetic is suitable.
Lateral anal sphincterotomy. In this operation, the internal sphincter is divided away from the fissure itself - usually either in the right or the left lateral positions. The procedure can be done by an open or a closed method. There does not seem to be any significant difference in outcomes between the open and closed techniques. Healing is usually complete within 3 weeks. The operation is more successful for acute than chronic fissures.
Fissurectomy and sphincterotomy. The essential part of the operation is to divide the transverse fibres of the internal sphincter in the floor of the fissure. If a sentinel pile is present, this is excised. The ends of the divided muscle retract and a smooth wound is left. Despite the presence of the wound, there is little or no pain and the results are good. The disadvantage of this operation is the prolonged healing time - usually not less than 3 weeks and often longer - and, occasionally, a mild, persistent and permanent mucus discharge. It is now reserved only for the most chronic or recurrent anal fissures, the majority being treated by lateral sphincterotomy.
Surgery clearly carries a risk of minor fecal incontinence, but topical treatments have problems with compliance, a lower rate of healing and a higher recurrence rate than surgical treatment. Even with the use of a good surgical technique there is the possibility of recurrence, and the patient must be warned as to an eventual discreet anal incontinence.
UNIT: COLOPROCTOLOGY
Theme: RECTAL PROLAPSE
KEY QUESTIONS FOR HOMEWORK:
Definition.
Pathogenesis.
Diagnosis.
Partial prolapse (characteristic and treatment in adults).
Complete prolapse (characteristic,differential diagnosis and treatment in adults).
Complications of the rectal prolapse.
1. Definition.
Rectal prolapse (procidentia) is a full thickness, circumferential intussusception of the entire rectal wall through the anal canal resulting in part of the rectum remaining intermittently or occasionally permanently distal to the anus. The latter condition is known as third degree prolapse and the former state as second degree. Internal intussusception is invagination of part or the entire rectum into itself without any external component.
Pathogenesis.
Etiologic factors: 1) congenital, 2) acquired.
Predisposing and associated anatomical and functional factors:
Anatomical factors include female sex, redundant rectosigmoid, deep pouch of Douglas, patulous anus (weak internal sphincter), diastasis of levator ani muscle (defects in pelvic floor), lack of fixation of rectum to sacrum.
Functional factors include poor bowel habits (chronic constipation), neurologic disease including congenital anomaly, cauda equina lesion, spinal cord injury, and senility.
The majority of patients are women and peak occurrence is in the sixth decade of life.
3. Diagnosis.
The patient will usually present with a protrusion (75%), and in about 70% of cases, coexisting fecal incontinence; almost 50% of patients have a history of constipation. The incontinence becomes more severe as the protrusion increases in degree. Dilatation of the canal by the mass results in further relaxation of the sphincter muscles and increased prolapse. Bleeding per rectum, discharge of mucus, or both, are common additional complaints. When an individual's symptoms are suggestive of rectal prolapse, having the patient sit on the toilet and bear down to feign evacuation is often the only means by which the rectal prolapse can be visualized. It is important to evaluate the tone and contractility of the sphincter mechanism. If sphincter tone is poor or if the anus is patulous, functional results after repair may be suboptimal. Alternatively, if the patient has relatively good sphincter tone and contractility, good bowel control can be ultimately anticipated. As occasionally a polyp or carcinoma of the rectum or sigmoid colon may be the "lead point" for an intussusception, an endoscopic examination should be performed. However ultimately, radiologic study of the rectum by means of defecography is most effective for identifying internal intussusception and other defecatory disorders.
4. PARTIAL PROLAPSE
A prolapse of only the rectal mucosa is called partial or incomplete or mucosal prolapse. The mucous membrane and submucosa of the rectum protrude outside the anus for approximately 1-4 cm. When the prolapsed mucosa is palpated between the finger and thumb, it is evident that it is composed of no more than a double layer of mucous membrane. There is some confusion as to its exact nature. Some believe that partial rectal prolapse represents the head of a rectal intussusception, and is the early manifestation of a complete rectal prolapse. Others consider it is a separate entity. The probable truth is that both types exist. The condition occurs most often at the extremes of life - in children between 1 and 3 years of age, and in elderly people.
In adults, the condition is usually associated with third-degree haemorrhoids. In the female, a torn perineum predisposes to prolapse, and in the male straining from urethral obstruction. In old age, both partial and complete prolapse are associated with atony of the sphincter mechanism but whether this is the cause of the problem or secondary to it is unknown.
Partial prolapse may follow an operation for fistula-in-ano where a large portion of muscle has been divided. Here the prolapse is usually localised to the damaged quadrant and is seldom progressive.
Prolapsed mucous membrane is pink; prolapsed internal haemorrhoids are plum coloured, and more.
Treatment in adults.
Submucous injections of sclerosants are successful in cases of early partial prolapse.
Excision of the prolapsed mucosa. When the prolapse is unilateral the redundant mucosa can be excised. When necessary, the operation is combined with haemorrhoidectomy.
5. COMPLETE PROLAPSE
Complete prolapse (syn. procidentia) is less common than the partial variety. The protrusion consists of all layers of the rectal wall and is a descending hernia-en-glissade of the rectum downward through the levator ani. As the rectum descends, it intussuscepts upon itself. A variant of complete rectal prolapse has been described in which the upper rectum prolapses into the middle or lower rectum without actually reaching the anal canal. This is called an internal prolapse, or intussusception of the rectum.
The process starts with the anterior wall of the rectum where the supporting tissues are weakest, especially in women. It is more than 4 cm and commonly as much as 10-15 cm in length. On palpation between the finger and the thumb, the prolapse feels much thicker than a partial prolapse, and obviously consists of a double thickness of the entire wall of the rectum. Any prolapse over 5 cm in length contains anteriorly between its layers a pouch of peritoneum. When large, the peritoneal pouch contains small intestine, which returns to the general peritoneal cavity with a characteristic gurgle when the prolapse is reduced. The prolapsed mucous membrane is often arranged in a series of circular folds. The anal sphincter is characteristically patulous and gapes widely on straining to allow the rectum to prolapse. Complete prolapse is uncommon in children. In adults, it can occur at any age, but is more common in the elderly. Women are six times more often affected than men. In women, prolapse of the rectum is commonly associated with prolapse of the uterus, or a past history of a gynaecological operation, e.g. hysterectomy. In approximately 50 per cent of adults, faecal incontinence is also a feature. The mucosa on the apex of the prolapse may show some granularity or even superficial ulceration from repeated trauma caused by contact with underclothing.
On reducing the prolapse it is important to carry out a sigmoidoscopic examination to exclude any other abnormality within reach of the instrument.
Differential diagnosis
Large third-degree haemorrhoids, a large polypoid tumour of the rectum, sigmoid colon prolapsing and emerging at the anus, or a purely mucosal prolapse must all be differentiated from a complete procidentia.
Treatment
Innumerable methods for fixing a rectal prolapse have been described in the surgical literature, and they can be divided into perineal, sacral and abdominal procedures. A procedure should be chosen with consideration for the age and fitness of the patient. There is perhaps no single ideal operation, and the art of prolapse management is to match the procedure to the patient. Whenever possible, an abdominal rectopexy is recommended, but when the patient is elderly and very frail, or is suffering from injury or disease of the spinal cord, or in very early life, a perineal operation is indicated.
Perineal approach.
Delorme's operation. In this procedure, the rectal mucosa is removed circumferentially from the prolapsed rectum over its length, apart from 0.5 cm strips at its proximal end and at its tip. A circular incision is made through the mucosa of the prolapse 1 cm from the dentate line. The underlying muscle is then imbricated with a series of chromic catgut sutures, such that when these are tied, the rectal muscle is concertinaed towards the anal canal. The anal canal mucosa is then sutured circumferentally to the rectal mucosaremaining at the tip of the prolapse. This manoeuvre has the effect of reducing the prolapse and creating a ring of muscle within the anal canal, which narrows the orifice and prevents recurrence. Delorme's procedure is a compromise operation and if recurrence does occur then the procedure can simply be repeated. It may also have a further advantage in that it does not cause constipation or an evacuation disorder, and there is no danger of pelvic nerve damage.
Thiersch operation. Incisions are made in front of and behind the anal margin to allow the passage of wire, stout nylon, or even silastic around the anal sphincter. In principle, the technique works by supporting the reduced prolapse and causing a local reaction which induces fibrosis and stenosis of the anal canal.
This procedure, which aims to place a steel wire, or more commonly now, a silastic or nylon suture, around the anal canal has in the past been the most frequently performed perineal procedure. However, it has become virtually obsolete for the treatment of rectal prolapse in adults,although it stilldoes have a place in the treatment of partial prolapse in children. The reasons for its lack of popularity are that the suture would often break or cause chronic perineal sepsis, or both, or the anal stenosis so createc would produce severe functional problems. Delorme's operation is now the preferred perineal operation.
Rectosigmoidectomy. With the patient in the lithotomy or jack-knife position the prolapse is pulled down, and the outer of the two tubes of rectal wall is divided circumferentially just above the dentate line. The inner tube of rectum can then be drawn down bringing the distal sigmoid to the anal canal, where it is divided and sutured to the anal remnant with absorbable sutures. The specimen resected should be 15 to 20 cm in length, leaving no slack rectum or distal colon to allow further prolapse. Popularity for this procedure has waned in the United Kingdom, but it is still very popular in the United States. Experience in the United Kingdom in the 1960s was disappointing with a high recurrence rate. Altermeier has modified the procedure to include suturing of the levator muscles anterior to the rectum. This is a plication of the puborectalis sling which attempts to improve sphincter function postoperatively and to reduce the incidence of recurrence.
If an abdominal repair must be avoided (e.g. in a young man in whom sexual potency must be preserved by avoiding damage to the pelvic nerves), more extensive perineal procedures are available. These include strengthening the puborectalis and external anal sphincters by an approach through the intersphincteric plane, the so-called postanal repair (Parks).
Abdominal approach. The principle of all abdominal operations for rectal prolapse is to replace and hold the rectum in its proper position. It is to repair the prolapse by mobilizing the rectum and fixing it to the sacrum. Of the many operations described, the following are relatively simple. They are recommended in patients with complete prolapse, who are otherwise in good health. Repair of the pelvic floor is sometimes performed simultaneously.
Wells' operation. In this operation the rectum is fixed firmly to the sacrum by inserting a sheet of polyvinyl alcohol sponge (or the Ivalon) between them. The rectum is separated from the sacrum in the usual way. The sponge is fixed by a series of sutures to the periosteum over the midline of the sacrum and is then wrapped loosely about the rectum covering all except the anterior wall. The free margins of the polyvinyl sponge are sutured to the lateral margins of the anterior wall of the rectum. The peritoneal floor is resutured so that the sponge is excluded from the peritoneal cavity. Polyvinyl sponge does not give rise to a foreign body reaction, but it does produce very marked fibrous tissue formation. Many proctologists regard this as the method of choice. Recently, the technique has been performed laparoscopically, thus reducing the operative trauma and limiting the time in hospital.
Ripstein's operation. In this operation, the rectosigmoid junction is hitched up by a Teflon sling to the front of the sacrum just below the sacral promontory. The mobilized rectum is fixed to the sacral hollow by means of a sling of Teflon mesh 5 cm wide. This is passed around the rectum and the ends are sutured behind it to the fascia on the front of the sacrum, just below the promontory. In addition, a few sutures are passed between the edges of the Teflon and the anterior and lateral rectal walls. The operation is very safe and simple and the results are good. Some surgeon's recommend combining this procedure with resection of the sigmoid colon.
Presacral rectopexy and simple suture. An alternative to the use of an implant of either Teflon or Ivalon is simply to mobilize the rectum and fix it to the sacral promontory. This technique is the popular operation in Russia.
Lohaut's operation. This operation depends entirely upon mobilising the rectum and lower sigmoid colon and holding it up by taking it through the rectus sheath. The results are moderate and few surgeons use this method.
It should be noted that approximately 50 per cent of adult patients with a complete rectal prolapse are incontinent, and rectopexy cures only about a third. Consequently, it may be necessary to perform a subsequent procedure to correct the incontinence.
Sacral procedures. A number of operations have been described which exploit the Kraske approach alongside the coccyx and behind the sacrum. With the patient in the jack-knife position an incision is made over the coccyx and parasacral area, giving access to the presacral and postrectal space. The rectum is mobilized, shortened by imbricating sutures, and foreign material is placed in the presacral space. None of these procedures has stood the test of time and become particularly popular.
6. Complications.
Most patients can reduce the prolapse readily, although urgent admission may be required if the bowel becomes oedematous and cannot be reduced. It may be possible to reduce such a prolapse manually in the clinic with the patient lightly sedated. In the rare situation of a gangrenous rectal prolapse an emergency perineal rectosigmoidectomy may be necessary. Proctitis, ulceration, and rarely severe haemorrhage can occur but these are not usually clinically important.
TESTS
1. The superior rectal artery arises from the -
a) Superior mesenteric artery
b) Inferior mesenteric artery
c) Internal illiac artery
d) Internal pudendal artery
2. Middle rectal artery is branch of......... artery :
a) Internal iliac
b) Internal pudendal
c) External iliac
d) Femoral
3. Fascia of Denonvilliers -
a) Membranous layer of fascia of the thigh
b) Perirenal fascia
c) Fascia between the rectal ampulla and the prostate and the seminal vesicles
d) Posterior layer of perirenal fascia
4. Muscle which is primarily responsible for rectal continence -
a) Ext. sphincter
b) Int. sphincter
c) Puborectalis
d) Sacrococcygeous
5. Internal sphincter of rectum is formed by -
a) Levator ani
b) Puborectalis
c) Longitudinal muscle fibres condensation
d) Circular muscles fibres condensation
6. Resting tone of rectum is decreased in all except –
Micturation
Retained faeces in the rectum
Prolapse rectum
Trauma involving the perineum
7. Sitz bath consists of which of the following -
a) Patient bathed in normal saline
b) Bathed in molten wax
c) Sits in a basin containing warm antiseptic lotion
d) Sits in a basin containing molten wax
8. Sentinel pile indicates - a) Carcinoma rectum b) Internal haemorrhoids
c) Perianal fistula
d) Anal fissure
9. Rectal examination should not be done -
a) Anal fissure
b) Fistula in anus
c) Prolapsed piles with bleeding
d) Anal stenosis
10. Commonest cause of rectal bleeding in a male between 20-40 yars is -
a) Carcinoma Rectum
b) Internal haemorrhoids
c) Fissure in anus
d) Rectal polyp
11. Commonest complication following haemorrhoidectomy is -
a) Haemorrhage
b) Infection
c) Faecal impaction
d) Urinary Retention
12. Treatment of choice in 2nd degree piles is -
a) Cryosurgery
b) Sclerotherapy
c) Banding
d) Surgery
13. Internal sphincterotomy is the treatment of choice for-
a) Piles
b) Fistula
c) Fissure-in-ano
d) Carcinoma
14. Treatment of primary piles is -
a) Surgery
b) Sclerotherapy
c) No treatment
d) Analgesics
15. Best investigation to diagnose piles is -
a) Proctosigmoidoscopy
b) Barium enema
c) Ultrasound
d) Proctoscopy
16. Commonest cause of rectal bleeding in India -
a) Cancer rectum
b) Internal haemorrhoids
c) Rectal polyps
d) Fissure-in-ano
17. The most common cause of painless rectal bleeding in a man is -
a) Fissure-in-ano
b) Diverticulosis
c) Piles
d) None
18. Five-day self subsiding pain is diagnostic of -
a) Anal fissure
b) Fistula-in-ano
c) Thrombosed external hemorrhoids d) Thrombosed internal hemorrhoids
19. The following are true of haemorrhoids except -
a) They are arteriolar dilatations
b) They are common causes of painless bleeding
c) They cannot be per rectally palpated
d) They can be handed
20. Commonest complication of cryotherapy is –
a) Bleeding
b) Persistent watery discharge
c) Pain
d) Ulceration
Answers
b
a
c
c
d
b
c
d
a
b
d
c
c
a,b
d
b
c
c
a
b
ГОУ ВПО «Смоленская государственная медицинская академия
Федерального агентства по здравоохранению и социальному развитию»
МЕТОДИЧЕСКИЕ УКАЗАНИЯ ДЛЯ СТУДЕНТОВ
ПО ДИСЦИПЛИНЕ хирургические болезни
ANORECTAL ABSCESSES AND FISTULAE IN ANO, PILONIDAL SINUS (DISEASE)
Составитель доц. Ю.И.Ломаченко
Методические указания утверждены на методическом совещании кафедры госпитальной хирургии (протокол № 2 от 6 октября 2008 г.)
Зав. кафедрой______________(проф. С.А.Касумьян)
2008 г.
UNIT: COLOPROCTOLOGY
Theme: ANORECTAL ABSCESS (SUPPURATION)
KEY QUESTIONS FOR HOMEWORK:
Definition.
Aetiology of anorectal abscesses.
Classification and clinical features.
Examination and differential diagnosis.
Treatment.
Prognosis.
1. Anorectal abscesses is a suppuration in tissue spaces surrounded the anal canal and rectum. Anorectal abscesses affect both sexes but are 2 to 3 times more common in men than women. Abscesses can occur at any age, but are most common in the fourth to the sixth decades of life.
2. Aetiology of anorectal abscesses.
In 60 per cent of cases the pus from the abscess yields a pure culture of Escherichia coli; in 23 per cent a pure culture of Staphylococcus aureus is obtained. In diminishing frequency, pure cultures of Bacteroides, a Streptococcus, or Proteus strain are found. In many cases the infection is mixed. In a high percentage of cases - some estimate it as high as 90 per cent - the abscess commences as an infection of an anal gland (a nonspecific infection of cryptoglandular origin). There are 6 to 10 such anal glands distributed around the anal canal which drain into the base of the anal crypts. Glands commonly ramify into the internal anal sphincter and can extend as far as the intersphincteric plane. Perianal infection may develop when a gland fails to drain adequately. If this results in abscess formation, then the communication with the anal canal often leads to involvement of either the internal anal sphincter or the intersphincteric plane (or both). Other causes of anorectal abscesses are penetration of the rectal wall, e.g. by a fish bone, a blood-borne infection, or an extension of a cutaneous boil. Underlying rectal disease, such as neoplasm and particularly Crohn’s disease, may be the cause. Similarly patients with generalised disorders, such as diabetes and more recently AIDS, may present with an anorectal abscess.
3. Classification and clinical features.
A clear understanding of suppuration in this area is dependent on a concise knowledge of the anatomy. There are four main varieties: perianal, ischiorectal, submucous, and pelvirectal. Infection originates in the intersphincteric plane, most likely in one of the anal glands. This may result in a simple intersphincteric abscess, or it may extend vertically either upward or downward, horizontally, or circumferentially, resulting in a number of clinical presentations.
An intersphincteric abscess is limited to the primary site of origin and may be asymptomatic or result in severe, throbbing pain that resembles the pain of a fissure. Pain persisting after adequate treatment of a coexisting fissure should raise suspicion of an underlying, unrecognized intersphincteric abscess.
Perianal abscesses (60 per cent). This is the most common abscess of the region. This usually occurs as the result of suppuration in an anal gland, which spreads superficially to lie in the region of the subcutaneous portion of the external sphincter. A perianal abscess results from the vertical downward spread of the intersphincteric infection to the anal margin. It may also occur as a result of a thrombosed external pile. If the haematoma is not evacuated, it may become infected and a perianal abscess results. Early diagnosis is made by inspecting the anal margin, when an acutely tender, rounded, cystic lump about the size of a cherry is seen and felt at the anal verge below the dentate line.
Ischiorectal abscess (30 per cent). Commonly, this is due to an extension laterally through the external sphincter of a low intermuscular anal abscess. Rarely, the infection is either lymphatic or blood borne. The fat, which fills the ischiorectal fossa, is particularly vulnerable because it is poorly vascularised. The ischiorectal fossa communicates with that of the opposite side via the postsphincteric space, and if an ischiorectal abscess is not evacuated early, involvement of the contralateral fossa is not uncommon («horseshoe» abscess). An ischiorectal abscess gives rise to a tender, brawny induration palpable on the corresponding side of the anal canal and the floor of the fossa. Constitutional symptoms are severe, the temperature often rising to 38°C-39°C. The patient may complain of pain and fever before an erythematous mass is detectable. Ultimately, an obvious red, fluctuant mass is visible.
Submucous abscess (5 per cent) occurs above the dentate line.
Pelvirectal (supralevator) abscess. If the infection spreads vertically upward, a supraelevator abscess may develop. These abscesses are difficult to diagnose because the patient may complain of vague discomfort, external manifestations are absent, and the presence of rectal induration and swelling may be clearly established only with the aid of an examination under anesthesia. Pelvirectal (supralevator) abscess is situated between the upper surface of the levator ani and the pelvic peritoneum. It is nothing more or less than a pelvic abscess and as such, is usually secondary to appendicitis, salpingitis, diverticulitis, or parametritis. Abdominal Crohn’s disease is an important cause of pelvic disease that can present as perianal sepsis.
Fissure abscess. This is the name given to a subcutaneous abscess lying in immediate association with an anal fissure.
4. Examination and differential diagnosis.
In all cases, it is important to establish whether there is a history of previous episodes of anorectal sepsis and how these were treated. Pain is a prominent initial feature of perianal and superficial ischiorectal abscesses, followed by local signs of inflammation. In the case of a perianal abscess, there is a localized, fluctuant, red, hot, and tender swelling close to the anus. Such symptoms are less evident or may even be absent with deep infections, which tend to develop insidiously with systemic upset (with generalized systemic signs such as fever, tachycardia, toxicity, leuocytosis). This can lead to diagnostic confusion. These patients are often diagnosed as fevers of unknown origin in outpatient clinics and emergency rooms and the actual diagnosis can be easily missed. Other features that might be noted are skin necrosis, if there is gross swelling, and crepitus if a gas-forming organism is present. Deeper infections produce are only apparent on digital rectal examination. The diagnostic clue in such instances is the presence of a tender mass or an area of induration. Fluctuance may be detected. It is important to ascertain the position of such lesions with respect to the gut tube and the pelvic floor as this can have an important bearing on subsequent management. Where doubt remains, endoluminal ultrasound (anal and rectal) as well as computerized axial tomography may be helpful. Locally invasive procedures such as sigmoidoscopy should be performed under general anaesthetic to prevent undue discomfort to the patient.
The only conditions with which an anorectal abscess is likely to be confused are an abscess connected with a pilonidal sinus, Bartholin’s gland, or Cowper’s gland. A perianal abscess can be misinterpreted as a thrombosed external hemorrhoid.
5. Treatment. As antibiotics cannot reach the contents of an abscess in adequate concentration, no reliance can be placed on antibiotic therapy alone. Operation should be undertaken early - as soon as it is certain that an abscess is present in this area - remembering that antibiotic therapy often masks the general signs. No time should be lost in evacuating the pus. A abscess should be treated in a timely fashion by incision and drainage. A specimen of pus should be sent for microbiological examination and the anal canal and abscess cavity should be examined for the presence of a fistula. Gentle pressure on the abscess may cause release of pus into the anal canal, demonstrating the site of an internal opening. Antibiotics are an unnecessary addition to routine incision and drainage of uncomplicated anorectal abscesses. Antibiotics should be considered in patients with highrisk conditions, such immunosuppression, diabetes, extensive cellulites.
An intersphincteric abscess is drained by dividing the internal sphincter to the level of the abscess.
Perianal abscesses. Perianal abscesses can most often be drained under local anesthesia. Thorough drainage is achieved by making a linear incision over the abscess. Healing commonly occurs within a few days.
Ischiorectal abscess. An ischiorectal abscess requires immediate, wide local drainage after incision through the skin and subcutaneous tissue overlying the infected space. The abscess cavity should be examined for possible extensions and, if septa exist, they should be broken down gently with a finger, and should be curetted and necrotic tissue excised (the necrotic tissue lining the walls of the abscess can be removed by the finger wrapped in gauze). For horseshoe abscess, the deep postanal space should be drained through a posterior midline incision extending from the subcutaneous portion of the external sphincter over the abscess to the tip of the coccyx, separating the superficial external sphincter and thus unroofing the postanal space and its ischioanal extension.
Submucous abscess is drained by incising the mucosa to the level of the abscess.
Pelvirectal (supralevator) abscess. An supralevator abscess requires immediate, wide local drainage after incision. The site of drainage will be dictated by the clinical findings, but should be placed over the most dependent part of the abscess, as close to the anal canal as possible.
Fissure abscess. Drainage is achieved at the same time as the fissure is treated by sphincterotomy.
6. Prognosis. A large percentage of anorectal abscesses coincide with a fistula in ano. For this reason, anorectal abscess becomes a highly important subject. Neglected abscesses can lead to devastating, necrotizing infections of the perineum that can spread with dramatic rapidity and become lethal.
UNIT: COLOPROCTOLOGY
Theme: FISTULA IN ANO
KEY QUESTIONS FOR HOMEWORK:
Definition.
Types of anal fistulas.
Special clinical types of fistulas in ano.
Clinical features.
Goodsall’s rule.
Investigation.
Treatment.
1. Definition.
Fistula-in-ano denotes the chronic phase of anorec-tal sepsis and is characterized by chronic purulent drainage or cyclical pain associated with abscess re-accumulation followed by intermittent spontaneous decompression. A fistula in ano is a track, lined by granulation tissue, which connects deeply in the anal canal or rectum and superficially on the skin around the anus. It usually results from an anorectal abscess (in up to 50 percent) which burst spontaneously or was opened inadequately. The fistula continues to discharge and, because of constant reinfection from the anal canal or rectum, seldom, if ever, closes permanently without surgical aid.
2. Types of anal fistulas.
The categorization of a fistula-in-ano is dependent on its location relative to the anal sphincter muscles according to Parks classification (Fig. – at next page):
intersphincteric – 45 percent. The fistula track is confined to the intersphincteric plane;
transsphincteric (which may be high or low) – 30 percent. The fistula connects the intersphincteric plane with the ischiorectal fossa by perforating the external sphincter;
suprasphincteric – 20 percent. Similar to transsphincteric, but the track loops over the external sphincter and perforates the levator ani;
extrasphincteric – 5 percent. The track passes from the rectum to perineal skin, completely external to the sphincteric complex.
The term "complex" fistula is a modification of the Parks classification, which describes fistulas whose treatment poses a higher risk for impairment of continence. An anal fistula may be termed "complex" when the track crosses >30 to 50 percent of the external sphincter (high transsphincteric, suprasphincteric, extrasphincteric).
Extrasphincteric fistulas – secondary to local disease. It occurs as a result of Crohn’s disease, ulcerative colitis, carcinoma, a foreign body perforating the rectal ampulla from above, or trauma.
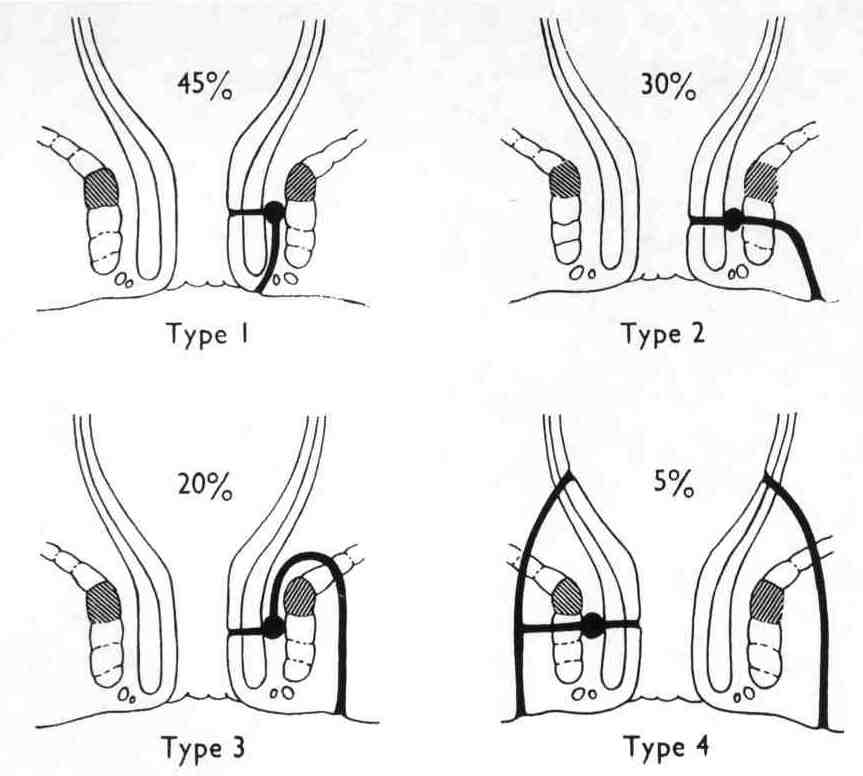
Figure. The four main anatomic types of fistulas: Type 1, intersphincteric; Type 2, transsphincteric; Type 3, suprasphincteric; Type 4, extrasphincteric. The external sphincter mass is regarded as the keystone, and the prefixes trans, supra, and extra refer to it. The puborectalis muscle has been cross-hatched for easy recognition.
3. Special clinical types of fistulas in ano.
Fistula connected with an anal fissure. Unlike the usual fistula in ano, pain (due to the fissure) is a leading symptom. The fistula is very near the anal orifice, usually posterior, and the external opening is often hidden by the sentinel pile.
Fistula with an internal opening above the anorectal ring is due, almost invariably, to penetration by a foreign body or probing and interference with a high anorectal (supralevator) abscess.
Fistula in patients with granulomatous infections and Crohn’s disease.
If induration around a fistula is lacking, if the opening is ragged and flush with the surface, if the surrounding skin is discoloured and the discharge is watery, or if the external openings are multiple, tuberculosis or Crohn’s disease should be considered. Fistulas with many external openings may arise from tuberculous proctitis, ulcerative proctocolitis, Crohn’s disease of colon or ileum, bilharziasis, and lymphogranuloma inguinale with a fibrous rectal stricture.
Crohn’s disease is the most frequent cause from this group. Crohn’s disease should also be suspected if there are other stigmata. A small and large bowel should be examined.
Tuberculosis. In more than 30 per cent of patients suffering from pulmonary tuberculosis, virulent tubercle bacilli are present in the rectum. If tuberculosis is suspected, a chest radiograph and sputum cultures are mandatory. However, the diagnosis can usually only be made on histological examination of biopsy material from the track. If due to tuberculosis, the fistula will usually respond to antituberculous drugs alone.
Carcinoma arising within perianal fistulas. In some instances, the fistulous condition, with its discharge of colloid material, overshadows the primary carcinoma, and not a few unfortunate patients have had their condition diagnosed for a time as an inflammatory fistula in ano. If a primary tumour is present in the rectum, usually it can be detected and its nature established by biopsy. Dukes established conclusively that colloid carcinomatous fistulas can develop without a primary neoplasm in the rectum. He regarded such cases as examples of colloid carcinoma developing in a reduplicated portion of the intestinal tract.
Hidradenitis suppurativa. This is a chronic infection of apocrine glands around the anal margin giving rise to numerous sinuses.
4. Clinical features.
Commonly, the principal symptom is a persistent seropurulent discharge that irritates the skin in the neighbourhood and causes discomfort. Often the history dates back for years. So long as the opening is large enough for the pus to escape, pain is not a symptom, but if the orifice is occluded pain increases until the discharge erupts. The primary orifice is usually at the dentate line where the infection originated. Frequently, there is a solitary external opening, usually situated within 3,5-4 cm of the anus, presenting as a small elevation with granulation tissue pouting from the mouth of the opening. Sometimes superficial healing occurs, pus accumulates and an abscess reforms and discharges through the same opening, or a new opening. Thus there may be two or more external openings, usually grouped together on the right or left of the midline but, occasionally, when both ischiorectal fossas are involved, an opening is seen on each side, in which case there is often intercommunication between them. As a rule there is much induration of the skin and subcutaneous tissues around the fistula.
5. Goodsall’s rule.
Fistulas with an external opening in relation to the anterior half of the anus tend to be of the direct type. Those with an external opening or openings in relation to the posterior half of the anus, which are much more common, usually have curving tracks, and may be of the horseshoe variety. Note that posteriorly situated fistulas may have multiple external openings which always connect to a solitary internal orifice, usually midline. The Goodsall’s rule is of little help in defining the anatomy of complex fistulas.
6.Investigation.
On examination. Subcutaneous induration may be traced from the external opening to the anal margin.
Digital examination. Not infrequently an internal opening can be felt as a nodule on the wall of the anal canal. Irrespective of the number of external openings, there is almost invariably only one internal opening.
Anoscopy may reveal the primary opening corresponding to the Goodsall's rule and occasionally compression of the track may express purulent material into the anal canal.
Proctoscopy sometimes will reveal the internal opening of the fistula. A hypertrophied papilla is suggestive that the internal orifice lies within the crypt related to the papilla.
Probing. A probe can be eased gently (not forcefully) from the external skin opening to the internal, anal canal opening. Such manoeuvres are painful, and are liable to reawaken dormant infection. Furthermore, if probing is performed without the utmost gentleness, or if the patient, experiencing pain, makes a sudden jerk, a false passage may result which complicates the condition still further. Probing should be postponed until the patient is under an anaesthetic in the operating theatre.
Fistulography using the injection of water-soluble contrast material into the track has been used with some success. Fistulography may be helpful in the investigation of the rare extrasphincteric fistula, but is rarely necessary in simpler fistulae. The procedure is likely to cause a recrudescence of inflammation.
Endoluminal ultra-sonography, CT and magnetic resonance imaging are being developed as techniques for «mapping» complex fistulas. CT and magnetic resonance imaging scanning have both been used in the assessment of fistula-in-ano but without any convincing evidence of improvement in management. Anal ultrasonography is not yet widely available, but can demonstrate secondary tracks and abscesses, and the use of hydrogen peroxide to show up bubbles in the fistula track which have a characteristic echo-rich appearance may improve its accuracy. Breaches in the internal sphincter and changes in the intersphincteric plane can be seen.
Treatment.
The goals in the treatment of fistula-in-ano are:
to eliminate the septic foci and any associated epithelialized tracks;
to do so with the least amount of functional derangement.
To initiate the most appropriate treatment, the etiology should be defined. This is usually cryptoglandular infection but may be related to Crohn’s disease, trauma or malignancy. There is no single technique appropriate for the treatment of all fistulas-in-ano and, therefore, treatment must be directed by the surgeon's experience and judgment.
Preoperative cleansing enemas are necessary.
Using bidigital palpation under anaesthesia, it is often possible to obtain more information concerning a fistula than can be learned from probing; it is surprisingly easy to push a probe through the wall of the track. Careful bidigital palpation of the perianal tissue will often reveal a cord-like induration, representing the track, which will lead the intra-anal finger towards the proximal opening. Rather than insert a probe through the distal orifice at this stage, it is better to endeavour to find the internal opening via a proctoscope. If the internal opening still cannot be seen, the insertion of a probe retrogradely into an anal crypt, especially one with a nearby hypertrophied papilla, often reveals the internal portion of the track. The internal opening can be identified by probing the external opening or by injecting a mixture of methylene blue and peroxide or other dye into the track. Methylene blue should be diluted so the color is a pale blue. Stronger concentrations not only color the adjacent tissue, but also obscure the fleshy redness of the fistula track granulation tissue. The mixture should be injected slowly and without too much pressure in order to avoid undue tissue dissection manifested by perianal or ischiorectal crepitus. The anatomy of most complex fistulas can be defined in the operating room without supplemental imaging studies. However, radio graphic evaluation may be a beneficial adjunct to identify occult internal openings, secondary tracts or abscesses, or to help delineate the fistula’s relationship to the sphincter complex. Magnetic resonance imaging and endorectal ultrasound are the studies of choice when radiologic assessment is deemed necessary.
Simple Fistula-in-Ano (intersphincteric and low transsphincteric).
Simple anal fistulas may be treated by fistulotomy. The fundamentals of fistulotomy – defining the entire fistula track from internal opening to external opening with identification and obliteration of primary and secondary tracks. Fistulotomy is preferable to fistulectomy. The latter results in larger wounds with a longer healing time and higher rates of incontinence.
Simple anal fistulas may be treated with track debridement and fibrin glue injection according to clinical practice guidelines of the American Society of Colon and Rectal Surgeons. Fibrin glue is an easy and repeatable treatment for fistula-in-ano with relatively few side effects and little to no risk of fecal incontinence. Successful healing rates from 60 to 70 percent can be achieved. Risk factors for failure include Crohn’s disease, rectovaginal fistula, human immunodeficiency virus, and short fistula length.
Complex Fistula-in-Ano.
Complex fistulas may be treated by the use of a seton and/or staged fistulotomy.
A seton – a time-honoured device – is a flexible foreign body (e.g., nonabsorbable soft braided suture material – a ligature of silk, linen or nylon) that is placed through the fistula track and secured to itself. There is some debate over the method of action of a seton: some clinicians feel that it works as a slow elastic ligature and therefore must be tightened periodically. Setons induce perisphincteric fibrosis along the fistula track so that when the fistulotomy is eventually performed, or the seton gradually tightened, the muscular defect and amount of incontinence is limited. A second school feel that the seton acts by ensuring chronic drainage of the fistula track. The seton acts as a wick/drain and allows the acute inflammatory reaction around the track to subside: this can greatly simplify subsequent surgery. A seton may also be utilized to facilitate staged fistulotomy. The seton is used to mark the external sphincter for later division after the subcutaneous components have healed. Although these two techniques have low recurrence rates (0-8 percent) according to clinical practice guidelines of the American Society of Colon and Rectal Surgeons, the rates for minor (34-63 percent) and major incontinence (2-26 percent) are significant.
Complex anal fistulas may be treated with endorectal advancement flap closure. The advantage of this procedure is that it does not require an sphincterotomy. The use of an endorectal advancement flap is an attractive modality for the treatment of a complex fistula-in-ano. It obliterates the septic focus and closes the internal opening, does not divide the sphincter, is repeatable, has a smaller wound, and can be combined with overlapping sphincter reconstruction for anterior fistulas. Success ful healing has been demonstrated in 55 to 98 percent of patients (data of the American Society of Colon and Rectal Surgeons). Although the sphincter mechanism is not divided during the construction of an endorectal advancement flap, minor incontinence has been reported in up to 31 percent of the patients and major incontinence in up to 12 percent. Predictors of poor outcome include undrained sepsis, cancer or radiation etiology, rectovaginal fistula diameter >2.5 cm, fistula present fewer than 6 weeks, and active Crohn’s proctitis.
Complex anal fistulas may be treated with debridement and fibrin glue injection according to clinical practice guidelines of the American Society of Colon and Rectal Surgeons. As with simple fistula-in-ano, fibrin glue is an easy, repeatable treatment for a complex fistula-in-ano. Using this technique, healing rates from 14 to 60 percent have been achieved in small studies.
The treatment of high-level fistulas is difficult. If the track is laid openas for low-level fistulas, incontinence will follow.
Supralevator fistula is quite unrelated to the ordinary type and the treatment is that of the cause.
A traumatic fistula usually needs a colostomy.
Crohn’s fistulas:
Asymptomatic fistulas need not be treated.
Simple, low Crohn’s fistulas may be treated by fistulotomy.
Complex Crohn’s fistulas may be treated with advancement flap closure if the rectal mucosa is grossly normal. Active proctitis is considered a contraindication.
Complex Crohn’s fistulas may be well palliated with long-term draining setons. The goal of a long-term loose (draining) seton for Crohn’s fistulas is to reduce the number of subsequent septic events by providing continuous drainage and preventing closure of the external skin opening.
Transsphincteric fistula with perforating secondary track. The condition starts as an intersphincteric track, often with a high secondary track in the ischiorectal fossa up to the levator ani. Here lies the danger. Although the anal opening may be low, during exploration of the high secondary track, unless great care is taken, the probe can be pushed through the levator ani into the rectal ampulla, thus converting a low fistula into a high-level type. Treatment should first of all be directed to the low transsphincteric fistula and healing of the upper track may follow. If it fails to do so, or if the opening into the rectum is of any size or near the anorectal bundle, a colostomy must sometimes be done before sound healing will take place. High tracks often require staged operations.
There is the other operative management of fistulas.
Dermal island flap anoplasty. It essentially includes raising an island of skin attached to subcutaneous fat to provide a blood supply to the flap. The inner edge of the flap is at the primary opening of the fistula and the outer edge includes the secondary opening of the fistula. No attempt is made to do a fistulotomy. Once the skin is raised, and the dissection may need to be at least 1 cm deep into the perianal flap to sliding the dermal flap into the anal canal, the primary opening is excised and all epithelial tissue is removed from this area. The internal sphincter is then closed transversely with a few absorbable sutures. The dermal flap is then slid into place and taking deep bites it is approximated to the cut edge of the rectal wall including smooth muscles in the bites, securing it with five or six sutures placed at the usual location of the dentate line. The external wound is left open to heal by secondary intention after securing hemostasis. This procedure is not universally performed and therefore many surgeons are not familiar with the technical detail of this effective and simple procedure. The success rate of this operation is around 80%.
The transphincteric approach is most suitable for extrasphincteric fistulas secondary to trauma. Dividing the sphincter mechanism posteriorly opens the rectum like a book, allowing approach to the fistula directly by excision and primary closure of the internal opening either in a single layer or as a vest over pants two-layer closure. Most surgeons are not familiar with this approach and are reluctant to divide the sphincter mechanism for fear of nonhealing and secondary fistula formation.
Always must be sent a piece of track for biopsy. A biopsy is taken for histology.
Postoperative care. Postoperative care is just as important as the operation.
Close follow-up and careful nursing of wound by a doctor and nurse team involve sitz baths, wound irrigation, and wound packing to ensure healing from the depth of the wound to the surface.
The seton may be removed 2 to 3 months later, at which time the track may heal spontaneously.
