
- •The characteristics of matter
- •Matter construction
- •Atomic structure
- •Matter is everything that has mass & volume.
- •Density
- •Melting point:
- •The boiling point is the temperature at which a substance begins to change from the liquid into the gaseous state.
- •Thermal conduction
- •Rusting of metals:
- •Complete the following statements:
- •Explain why:
- •Problems :
- •What’s meant by:
- •Melting point
- •A solid substance (ice) melts when heated.
- •A liquid (water) evaporates when heated.
- •Vaporization is the change of liquid into gas.
- •Explain why:
- •Write the scientific term:
- •Match the figure with the following molecules:
- •Match the figure with the process:
- •Fill in the blanks:
- •Give reasons:
- •Choose the right answer:
- •Figure………………………is an excited atom while, figure…………………is for the atom in the ground state.



Lesson
1 : Lesson
3: Lesson
4:The characteristics of matter
Matter construction
Atomic structure



Matter is everything that has mass & volume.
Air, Water are example of matter.
Different kinds of matter differ in their characteristics.
Water & vinegar have different tastes.
Colour, taste & smell help us to distinguish different substances.
Density
Activity 1:
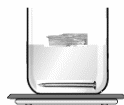 Put
a nail, the piece of wood, some oil & an ice cube in a beaker of
water.
Put
a nail, the piece of wood, some oil & an ice cube in a beaker of
water.
Observation :
The nail sinks in the water.
The ice , the piece of wood & oil float on the surface of water.
Conclusion:
The nail is denser than water, therefore it sinks.
The ice , candle & oil are less dense than water & therefore they float.
Density is the mass of a
unit volume (1 cm3)

1 cm3 Iron
Mass
= 7.87g
1 cm3 Water
Mass
= 1g
1 cm3 Oil
Mass
= 0.82g
D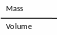 ensity
is calculated by the rule
ensity
is calculated by the rule
Density =
|
Mass |
Volume |
Density |
Unit |
gm |
cm3 |
g/ cm3 |
A solved example:
An object’s mass is measured in the lab. & found to be 50g.
The volume was also measured & found to be 25 cm3.
Can you find through calculations if this object floats or sinks in water.
S olution:
olution:
D ensity
=
ensity
=
= = 2 g/ cm3
The density of this object is bigger than water 1 g/ Cm3 therefore it sinks.
E xplain
why:
xplain
why:
Balloons filled with helium rise upwards. That’s because the density of helium is less than that of air.
Burning oil & flaming petroleum oil aren’t extinguished with water.
The density of oil is lower than water, therefore oil floats on top of water & keeps burning.

Melting point:
Melting is the change of the state of an object from solid to liquid.
Melting needs heating to occur.
The temperature at which melting of a substance begins is called melting point.
Metals such as iron, copper & aluminium have high melting points.
Ice, wax & butter have low melting points.
Metals have high melting points.
Explain why:
Metals are molten to make machines from them.
B ec.
Molten metals are easily shaped or mixed into alloys.
ec.
Molten metals are easily shaped or mixed into alloys.
Cooking pots are made of stainless steel alloy.
Bec. Both aluminum & stainless steel are good conductor of heat and
have high melting points.Stainless steel also doesn’t rust when water is heated inside it.

An alloy is a mixture of 2 metals or more, metals are mixed to improve their characteristics.
E xamples are :
Gold & copper alloy used in making jewellery.
N
 ickel
chrome alloy used in making the heating coils of electric heaters &
boilers.
ickel
chrome alloy used in making the heating coils of electric heaters &
boilers.
The boiling point is the temperature at which a substance begins to change from the liquid into the gaseous state.
Different substances have different boiling points.
Electric conduction
|
Good electric conductors |
Bad electric conductors |
Definition |
They allow electricity to pass through them. |
They don’t allow electricity to pass through them. |
Examples |
M
Some acid, alkali or salt solutions. |
N on – metals such as sulphur & phosphorus. Sugar solution H ydrogen chloride solution in benzene. wood & plastic. |
Uses |
Electric cables are made of copper Electric screwdrivers are made of steel. |
H |

 etals
such as copper & silver.
etals
such as copper & silver.