
- •Lesson (1) Chemical structure of living organisms' bodies (Carbohydrates and Lipids)
- •For Reading only (Enriching informations)
- •Lesson (2) Chemical structure of living organisms' bodies (Proteins and nucleic acids)
- •Lesson (3) Water
- •Importance of this property
- •Importance of this property
- •Importance of these properties
- •Importance of this property
- •Importance
- •Lesson (4) Chemical reactions inside organisms' bodies
- •Answer only four questions
Lesson (2) Chemical structure of living organisms' bodies (Proteins and nucleic acids)

![]()
Proteins form the bodies of all living organisms, they also take part in the biological reactions occurring within living organisms which help them sustain life.
The importance of proteins
1 - The basic component of cell membranes
, ligaments and tendons 2 - They form muscles, fingernails, hair, organs, glands
3- They form liquids in human body such as lymph and blood
4- They are necessary for human growth
5- The main component of chromosomes
6- They form enzymes and hormones
hooves and horns of animals, and spider webs are formed from proteins
The molecular structure of proteins
Polymers of proteins are composed of monomers called "amino acids"
Amino acids:-
The building units of proteins, they are organic compounds which consist of carbon, oxygen, hydrogen and nitrogen atoms.
Structure: An amino acid is composed of a carbon atom linked with:-
- An acidic functional group called amine NH2
- A basic functional group called carboxyl COOH
- R Group (side group) which differs according to the type of amino acid

Fig. (4) The structure of amino acids
Amino acids and building proteins
Proteins are formed from groups of amino acids linked together by peptide bonds
Peptide bond: A bond between two molecules which is formed when the carboxyl group of an amino acid reacts with the amine group of another one releasing water molecule (H2O)
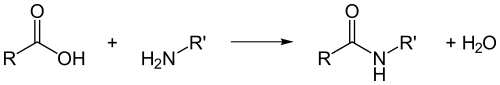
Fig. (5) How peptide bonds form
Two amino acids linked by peptide bond are called Dipeptide, while a protein chain formed from many amino acids linked by peptide bonds is called polypeptide
Proteins are formed from the same 20 amino acids, but with different arrangements.
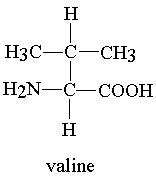
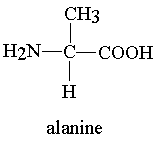
Example of amino acids:-
1- Alanine
2- Glycine
3- Valine
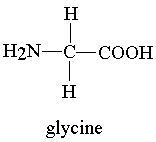
How to detect proteins in substances
We detect proteins by using Biuret reagents, proteins change the colours of these reagents from blue to purple
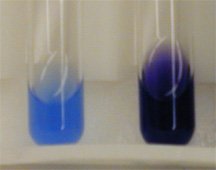
Fig. (6) Biuret reagents
The classification of proteins
Proteins are classified according to their structure into:-
Simple proteins:-
Structure: They consist of only amino acids
Examples: Albumin, which is found in blood plasma, leaves and seeds of plants
Associated proteins:-
Structure: They consist of amino acids associated with other elements.
Examples:-
1- Nuclear-associated proteins:
2- Phosphoproteins: They contain phosphorus element (ex. Casein – milk protein)
3- Thyroxin: Hormone secreted by thyroid gland and contains iodine element.
4- Blood hemoglobin: Its protein contains iron element.
![]()
Primary structure of protein:-
It describes the arrangement of amino acids in polypeptides of a certain protein.
This level determines the no. , kind and the arrangement of the amino acids forming protein.
Secondary structure of protein:-
It describes the way by which polypeptides are coiled.
This structure is formed due to the hydrogen bonds between carboxyl and amine groups in close amino acid monomers.
Tertiary structure of protein
It describes the three-dimensional shape of proteins.
This structure is formed due to the bonds between the side groups (R groups) of amino acids which bends the different polypeptide chains which gives protein its unique shape.
Quaternary structure of protein
It describes proteins which consist of two or more chains of polypeptide.
This structure is formed due to the linkage of polypeptide chains with each other.
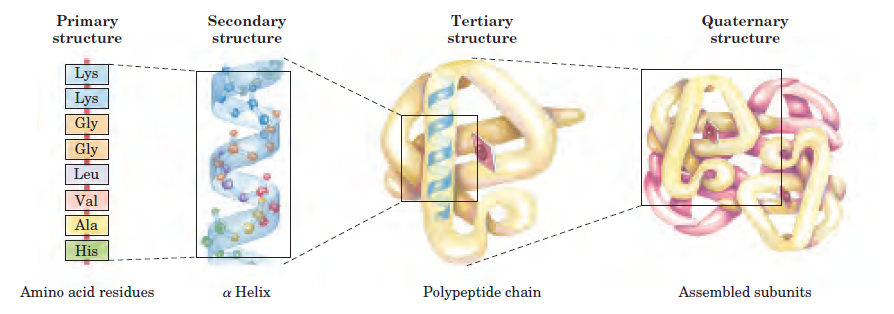
Fig. (7) Levels of protein structure
![]()
They are biological macromolecules which consist of hydrogen, oxygen, carbon, nitrogen and phosphorus. Nucleic acids have two kinds which are:-
- Ribonucleic acid (RNA)
- Deoxyribonucleic acid (DNA)
Nucleic acids consist of structural units called nucleotides which are bound together by covalent bonds forming polynucleotide
Nucleotides:-
The building units of nucleic acids, each one of them is composed of three units which are:-
- Pentose (5 carbon) sugar:
There are two types of pentose sugar which form nucleic acids, these types are:-
Ribose: forms RNA
Deoxyribose: forms DNA
- Phosphate group
It's linked by a covalent bond to the carbon atom no. 5 of sugar molecule of the nucleic acid.
- Nitrogenous base
There are five nitrogenous bases which are:-
- Adenine (A)
- Thymine (T) Replaced by Uracil (U) in RNA molecules
- Cytosine (C)
- Guanine (G)
Each of the previous bases link with the 1st carbon atom of sugar molecule in covalent bond.
Nucleic acids differ according to their pentose sugars and nitrogenous bases
Uracil base in RNA molecule is the equivalent to thymine base in DNA molecule.
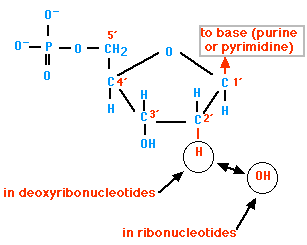
Fig. (8) The structure of a nucleotide
The importance of DNA
1- It is from the basic components of chromosomes.
2- It is responsible for transferring hereditary traits through generations.
3- It carries the hereditary information responsible for the characteristics of living organisms and organization of the biological processes within cells.
DNA molecule consists of two strands coiled around each other.
Guanine base binds to Cytosine base with triple hydrogen bond.
Thymine base bind to Adenine base with double hydrogen bond
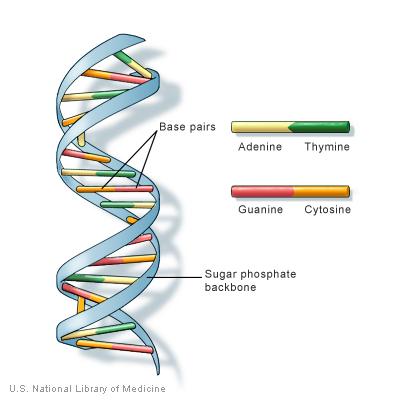
Fig. (9) The structure of DNA molecule
The importance of RNA
It copies the information of DNA , then it transports to cytoplasm to be used in making proteins which are responsible for the hereditary traits and organization of biological processes.
Guanine base binds to Cytosine base with triple hydrogen bond.
Uracil base bind to Adenine base with double hydrogen bond.
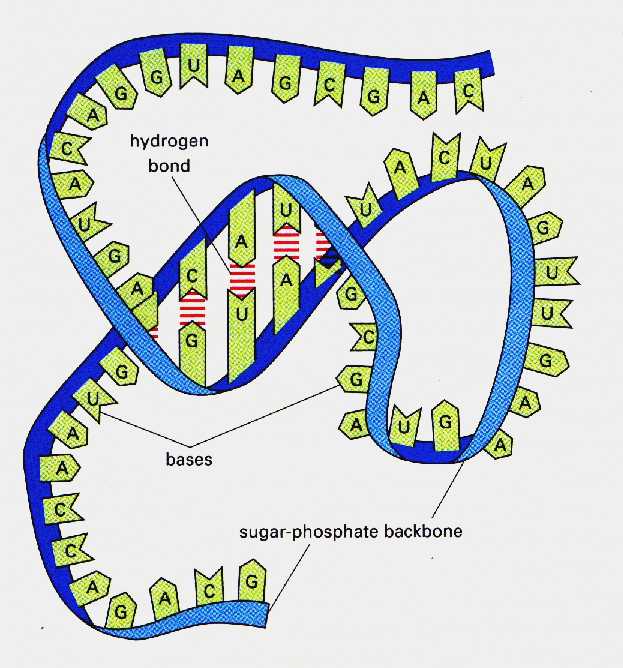
Fig. (9) The structure of RNA molecule
![]()
Proteins: They are complex biological macromolecules which consist of oxygen, hydrogen and carbon atoms basically, they have heavy molecular weights, and their structural units (monomer) are amino acids.
Amino acids: The building units of proteins, they are organic compounds which consist of carbon, oxygen, hydrogen and nitrogen atoms.
Peptide bond: A bond between two molecules which is formed when the carboxyl group of an amino acid reacts with the amine group in another one, which releases water molecule (H2O)
Dipeptide: Two amino acids linked by peptide bond.
Polypeptide: protein chain formed from many amino acids.
Nucleic acids: They are biological macromolecules which consist basically of hydrogen, oxygen, carbon and nitrogen. They are composed of structural units called nucleotides. Nucleic acids divide into RNA and DNA.
![]()
1- Proteins play an important role in life.
Because :-
1 - They are from the basic component of cell membranes
, ligaments and tendons 2 - They form muscles, fingernails, hair, organs, glands
3- They form liquids in human body such as lymph and blood
4- They are necessary for human growth
5- The main component of chromosomes
6- They form enzymes and hormones
2- The importance of DNA
Because:-
1- It is from the basic components of chromosomes.
2- It is responsible for transferring hereditary traits through generations.
3- It carries the hereditary information responsible for the unique characteristics of living organisms and organization of the biological processes within cells.
3- The importance of RNA
Because it copies the information of DNA , then it transports to cytoplasm to be used in making proteins which are responsible for the hereditary traits and organization of biological processes.
4- The importance of the primary structure of proteins
Because it describes the arrangement of amino acids in polypeptides of a certain protein and determines the no. , the arrangement and kind of the amino acids forming proteins.
5- The importance of the secondary structure of proteins
Because it describes the way by which polypeptides coil around themselves.
6- The importance of the tertiary structure of proteins
Because it describes the three-dimensional shape of proteins.
7- The importance of the quaternary structure of proteins
Because it describes proteins which consist of two or more chains of polypeptide.
8- When albumin protein disassociate, it produces amino acids only.
Because albumin is a simple protein which consists of only amino acids.
9- There are millions of different proteins although they are formed from the same 20 amino acids.
Because proteins structures differ by the difference of the arrangement of the amino acids forming them.
![]()
1- Choose the correct answer
1- ……. are the basic components of lymph and blood in human body.
A- Proteins B- Carbohydrates C- Lipids D- Nucleic acids
2- Polymers of proteins are composed of monomers called …….
A- Nucleic acids B- Amino acids C- Citric acids D- Uric acid
3- The acidic functional group forming an amino acid is called…….
A- Amine B- Carboxyl C- Hydroxyl D- Phosphate
4- The basic functional group forming an amino acid is called…….
A- Amine B- Carboxyl C- Hydroxyl D- Phosphate
5- Proteins are formed from groups of amino acids linked together by ……. Bond
A- Ionic B- Hydrogen C- Hydrophobic D- Peptide
6- …………. is from simple proteins.
A- Albumin B- Casein C- Thyroxin D- Hemoglobin
7- ……….. is from phosphoproteins.
A- Casein B- Hemoglobin C- Albumin D- Insulin
8- Casein is a protein which contains ……… element
A- Iron B- Iodine C- Phosphorus D- Sulphur
9- Hemoglobin protein contains ……… element
A- Iodine B- Phosphorus C- Sodium D- Iron
10 - Thyroid gland secretes thyroxin hormone, the proteins of thyroxin hormone contain……. element
A- Iodine B- Phosphorus C- Calcium D- Potassium
11- ………. carries the hereditary traits of living organisms.
A- DNA B- RNA C- Amino acid D- Phosphoric acid
12- ……… acid helps in protein synthesis process which cause the appearance of hereditary traits
A- Ribonucleic B- Deoxyribonucleic C- amino D- Sulphuric
2- Write the scientific term
1- Biological macromolecules whose monomers are called amino acids.
2- A bond between two molecules form when the carboxyl group of an amino acid reacts with the amine group in another one, which releases water molecule (H2O)
3- Two amino acids linked by peptide bond.
4- protein chain formed from many amino acids.
5- They are composed of structural units called nucleotides.
6- A kind of protein found in milk
7- Protein which consist of only amino acids.
8- Proteins which consist of amino acids associated to other elements.
9- Protein found in blood and consists of amino acids bound to iron element
10- The building units of nucleic acids
11- A kind of pentose which forms the nucleotides of RNA
12- A kind of pentose which forms the nucleotides of DNA
13- The nucleic acid which is responsible for transferring hereditary traits through generations.
14- The nucleic acid which takes part in making proteins which are responsible for the hereditary traits and organization of biological processes.
3- Write short notes about
1- Importance of proteins
2- Importance of DNA
3- Importance of RNA
![]()
1- Choose the correct answer
1- Proteins
2- Amino acids
3- Carboxyl
4- Amine
5- Peptide
6- Albumin
7- Casein
8- Phosphorus
9- Iron
10- Iodine
11- DNA
12- Ribonucleic
2- Write the scientific term
1- Proteins
2- Peptide bond
3- Dipeptide chain
4- Polypeptide chain
5- Nucleic acids
6- Casein proteins
7- Simple proteins
8- Associated proteins
9- Hemoglobin
10- Nucleotides
11- Ribose
12- Deoxyribose
13- DNA
14- RNA
