
- •Lesson (1) Chemical structure of living organisms' bodies (Carbohydrates and Lipids)
- •For Reading only (Enriching informations)
- •Lesson (2) Chemical structure of living organisms' bodies (Proteins and nucleic acids)
- •Lesson (3) Water
- •Importance of this property
- •Importance of this property
- •Importance of these properties
- •Importance of this property
- •Importance
- •Lesson (4) Chemical reactions inside organisms' bodies
- •Answer only four questions
![]()
Lesson (1) Chemical structure of living organisms' bodies (Carbohydrates and Lipids)
We studied before that living organisms' bodies consist of:-
Systems Organs Tissues Cells Cellular organelles
Cells of living organisms consist of organic molecules and inorganic molecules.
Organic molecules in living organisms are carbohydrates, lipids, proteins and nucleic acids. They are big molecules containing hydrogen and carbon basically
Such big biological molecules are also known as "Biological macromolecules"
Inorganic molecules in living organisms are water and salts, which may contain carbon or not.
![]()
They are huge biological molecules composed of smaller molecules called monomers. Biological macromolecules are also called Polymers
Monomers bind together forming polymers by a polymerization process
![]()
They are biological macromolecules which consist of hydrogen, oxygen and carbon. They include sugars, fibres and starches and their general formula is (CH2O)n (which means that they consist of carbon, hydrogen and oxygen at ratio 1:2:1 respectively)
The importance of carbohydrates:-
1- The main and quickest source of energy in living organisms
2- They are used in storing energy in living organisms till they need it, as plants store carbohydrates in the form of starch, whereas animals and humans store them in the form of Glycogen in liver and muscles
3- The basic component of some parts of cell such as cellulose in the cell walls of plant cells, protoplasm and cellular membranes
The molecular structure of carbohydrates
Carbohydrates are divided according to their structures into:-
Simple sugars:-
A Simple sugar whose polymers consist of only one molecule is called Monosaccharide while that which consist of 2 molecules is called Disaccharides
Common properties of simple sugars:-
1- Soluble in water
2- They have small molecular weights
3- They have sweet tastes
How to detect simple sugars in food
We can detect simple sugars in food by using Benedict reagent, simple sugars change its colour from blue to orange.

Fig. (1) Benedict reagent
Monosaccharides:-
The simplest kind of sugars whose polymer consist of only one molecule
Structure: A molecule composed of a series of carbon atoms, each one of them is bound with an oxygen atom and a carbon one in a certain way.
The no. of carbon atoms in a monosaccharide ranges from 3 to 6 atoms
Examples:-
1- Glucose (Grapes sugar)
2- Fructose (Fruits sugar)
3- Ribose
Disaccharides:-
Structure: Two molecules of monosaccharides bound together
Examples:-
1- Sucrose (sugar cane): It consists of glucose molecule bound with fructose one
2- Lactose (milk sugar): It consists of glucose molecule bound with Galactose one
3- Maltose (malt sugar): It consists of two bound glucose molecules
Monosaccharides role in energy transfer processes inside living organisms:-
Living organisms release the energy stored in monosaccharides such as glucose as the following:-
1- Glucose is oxidized inside mitochondria in cells
2- The energy stored in glucose gets released in the form of chemical bonds
3- The chemical bonds are stored in compounds called Adenosine Triphosphate ( ATP)
4- ATP transports to other parts of cell using its stored energy in all the biological processes in cell
Complex sugars (Polysaccharides)
They are carbohydrates which consist of group of monosaccharides, Complex sugars are also called Polysaccharides
Common properties of simple sugars:-
1- Insoluble in water
2- They have heavy molecular weights
3- They don't have any taste
Examples:-
1- Cellulose
2- Starch
3- Glycogen
(N.B: The polymers of each one of the previous complex sugars consist of glucose molecules bound together)
How to detect starch in substances
Starch changes the colour of iodine solution to blue
![]()
They are biological macromolecules which are composed of carbon, oxygen and hydrogen. They divided into simple lipids (fats, waxes, oil) , complex lipids (phospholipids) and derivative lipids (steroids)
Lipids dissolve in non polar solvents such as carbon tetrachloride and benzene, but don't dissolve in polar solvents such as water
How to detect lipids in substances
Sudan IV reagent is used to detect lipids, as lipids can dissolve in it changing its colour to red
The molecular structure of lipids
Lipids are formed from 3 fatty acids bound to a glycerol molecule
glycerol is an alcohol having 3 hydroxyl OH groups
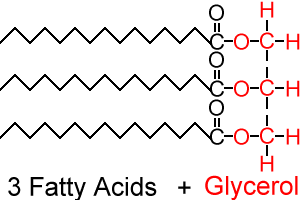
Fig. (2) the structure of lipids
The importance of lipids
1- A source of energy: The energy released from lipids is more than that released from carbohydrates. Human body begins releasing energy from lipids when it runs out of carbohydrates.
2- The main component of cell membranes.
3- They make up 5% of the organic compounds forming living cells.
4- Some animals (polar bears, penguins, seals) store lipids under their skins to protect them from low temperatures.
5- They work as protective layers in some plants and animals.
6- Some of them (steroids) work as hormones
The classification of lipids
Simple lipids:-
They are formed from the reaction of fatty acids with alcohols, they are classified according to the saturation of fatty acids into oils, fats and waxes.
Oils (Triglycerides)
They are liquid lipids formed from the reaction of unsaturated fatty acids with glycerol.
Some birds' feathers are covered with oils to protect them from water which disable their movement.
Fats (Glycerides)
They are solid lipids formed from the reaction of saturated fatty acids with glycerol.
Waxes
They are formed from the reaction of fatty acids with heave molecular weights with monohydroxy alcohols (alcohols having one hydroxyl OH group).
Wax covers the leaves of plants (especially desert plants) to decrease the amount of water they lose by transpiration process.
