
- •Mr. Ahmed mabrouk second term 2011
- •1(A Metal substitutes water hydrogen or acid:
- •2) The substitution of a metal instead of another one into its salt
- •1) Reaction between acid and Alkali. (Neutral)
- •2) The reaction of acid with salt
- •3 ) The Reaction of salt with salt
- •L esson 1 exercises
- •L esson 2: Speed of Chemical reactions
- •The definition of the speed of chemical reaction
- •The speed of chemical reaction:
- •Type of coherence in reactants
- •C atalysts have some common properties:
- •Biology
- •L esson 2 Exercise
- •Lesson 3: Solution
- •Solutions of acids, bases and minerals
- •Economic importance of some common acids
- •E conomic importance of some common bases
- •Economic importance of some common minerals
- •Lesson 3 Exercise
- •L esson 3 Exercise
- •Science, Technology and Society
- •A) Shinning metal
- •B) In the Kitchen
- •C) In the garden
- •D) In the medical field
- •Electromotive force ( emf )
- •T ypes of electric resistance:
- •The relationship between the current intensity and the potential difference (Ohm’s law):
- •Lesson 1 exercises
- •1 Complete the following sentences:
- •2 Choose the correct answer for each of the following statements:
- •3 Write the scientific term corresponding to each of the following statements:
Lesson 3: Solution

Examples of Solutions:
- Tea - juices – soup – Blood - perfumes - paints.
The mixture of both water and salt is homogenous in all its parts and called a solution.
Solution is the mixture that is homogenous in composition and properties.
It consists of two or more substances that are not chemically united (solute & solvent).
Solvent is the substance with the greater amount in the solution.
Solute: is the substance with the smaller amount in the solution.
Solutions are classified in different types in terms of concentration, homogeny and size of molecules as follows:

Activity: Types of solutions in terms of homogeny
Steps:
1- Put some pure water in each glass of 3 glasses.
2- Put a spoon of salt in the first glass, a spoon of sand in the second and a spoon of oil in the third.
3- Stir each glass.
Observation:
Solutions can be classified in terms of homogeny into a homogenous solution and a non-homogenous solution.
Homogenous solution:
It is the solution in which the solute molecules are distributed in the solvent in a regular way in all its parts and they can not be distinguished.
The solution is characterized by the same appearance in all its parts like the sugar solution and table salt solution.
Non - homogenous solution:
It is the solution in which the solute molecules are distributed in the solvent in an irregular way in all its parts as there is a contrast in the properties of its parts that can be distinguished be the naked eye. It consists of two or more layers.



Activity: Types of solutions in terms of concentration of solute
Procedures:
1- Pour 100ml distilled water in the glass.
Put a little table salt in the glass and stir well.
Observation: Salt dissolves in water forming unsaturated solution.
3- Continue adding more table salt with stirring so that no more additional amounts of table salt can be dissolved in water.
Observation: Salt doesn't dissolve in water forming saturated solution.
4- Heat the glass and observe what happens to the precipitated table salt.
5- Add additional amounts of table salt and continue heating.
Observation: The additional amounts of table salt dissolve forming pre-saturated solution.
6- Get the glass away from the flame and put a little table salt in the tube.
Observation: Table salt doesn't dissolve.

Solutions can be classified in terms of the solute |
|
|
|
|
2- Saturated solution |
|
|
(No. of dissolved molecules is equal to No. of the precipitated molecules). |
|
|
|
|
|
|
|
|
(the amount of the solute is greater than in the case of the saturated solution). |

Activity: types of solutions in terms of the size of the solute molecules
Tools: Three glasses (salt solution in water – chalk solution in water – milk)
Steps: 1- Observe the three glasses by the naked eye.
2- Can you distinguish the solute particles by the naked eye?
3- Stir the three solutions well and leave them for a period of time.
 4-
Put a filter paper in a funnel then put the funnel in an empty glass
and pour each of the three solutions separately.
4-
Put a filter paper in a funnel then put the funnel in an empty glass
and pour each of the three solutions separately.
5- Observe what remains on the filter paper.
Observation & conclusion:
S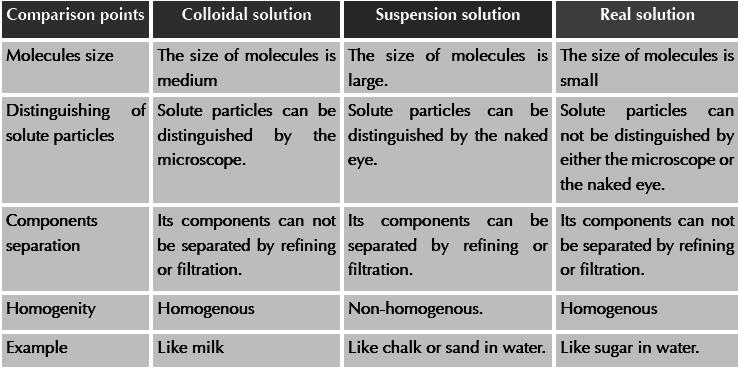 olutions
can be classified in terms of the size of the solute molecules into
three types
olutions
can be classified in terms of the size of the solute molecules into
three types
Unit 1: Parts of Lesson 3: Solution

