
- •Mr. Ahmed mabrouk second term 2011
- •1(A Metal substitutes water hydrogen or acid:
- •2) The substitution of a metal instead of another one into its salt
- •1) Reaction between acid and Alkali. (Neutral)
- •2) The reaction of acid with salt
- •3 ) The Reaction of salt with salt
- •L esson 1 exercises
- •L esson 2: Speed of Chemical reactions
- •The definition of the speed of chemical reaction
- •The speed of chemical reaction:
- •Type of coherence in reactants
- •C atalysts have some common properties:
- •Biology
- •L esson 2 Exercise
- •Lesson 3: Solution
- •Solutions of acids, bases and minerals
- •Economic importance of some common acids
- •E conomic importance of some common bases
- •Economic importance of some common minerals
- •Lesson 3 Exercise
- •L esson 3 Exercise
- •Science, Technology and Society
- •A) Shinning metal
- •B) In the Kitchen
- •C) In the garden
- •D) In the medical field
- •Electromotive force ( emf )
- •T ypes of electric resistance:
- •The relationship between the current intensity and the potential difference (Ohm’s law):
- •Lesson 1 exercises
- •1 Complete the following sentences:
- •2 Choose the correct answer for each of the following statements:
- •3 Write the scientific term corresponding to each of the following statements:
C atalysts have some common properties:
They change the speed of the reaction but do not affect its beginning or its end.
No chemical changes or decrease in mass occur to the catalyst before or after the reaction.
They are combined to reactants but get separated from them quickly to form the products (resultants) at the end of the reaction.
They decrease the energy needed for the reaction.
A small amount of catalyst is often enough to complete the reaction.

Activity: The breaking up of hydrogen peroxide
Procedures:
Put an equal amount of hydrogen peroxide in the two test tubes.
Put a small amount of manganese dioxide in tube (A(.
Observation:
Hydrogen peroxide in tube (A) releases more oxygen bubbles.
Conclusion: Manganese dioxide helps in the increase of speed of breaking up of hydrogen peroxide.

A ctivity:
Effect of enzymes on speed of chemical reaction
ctivity:
Effect of enzymes on speed of chemical reaction
Procedures:
Fill a half of the glass with hydrogen peroxide.
Put the piece of the sweet potato in the glass.
Observation:
Hydrogen peroxide produces more oxygen bubbles after putting sweet potato.
Conclusion: Oxidize enzyme in potato helps in breaking up of hydrogen peroxide.
Biology
The human body contains thousands types of enzymes.
Each type has a specific function. Without enzymes man can never breathe, move, or digest food.
A molecule of one enzyme can do its function million times per minute.
The reaction occurs in the presence of enzymes more rapidly than that without the enzymes thousands or millions times.
L esson 2 Exercise
A- Complete the following sentence:
At the beginning of the reaction, the concentration of reactants is…………….. %
The change in the concentration of reactants and resultants in a time unit is...................................
The increase in concentration of reactants makes the chemical reaction......................
The reaction of contributing (covalent) compounds is .................................
Sodium chloride powder reacts ................................. than a cube of sodium chloride.
A substance which increases the speed of chemical reaction without sharing in the reaction is .............................
B- Give reasons for:
1- The fridge is used to preserve food.
………………………………………………………………………………………..
2- Using Molecule nickel النيكل المجزأ)) in hydrating oil instead of pieces of nickel.
………………………………………………………………………………………...
3- Reactions between ionic compounds are fast whereas reactions between contributing compounds are slow.
………………………………………………………………………………………...
………………………………………………………………………………………...
4- The speed of the chemical reaction increases when the amount of the reactants increases.
………………………………………………………………………………………..
C- The following equation explains the breaking up of a compound:
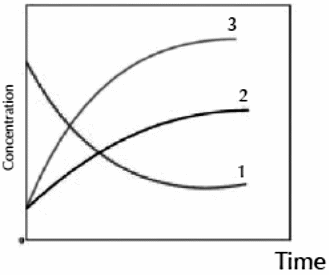 2X
2Y + Z
2X
2Y + Z
The following graph illustrates the change in concentration of reactants and resultants in respect to time.
Write the name of compound which each number indicate.
……………………………………………….
……………………………………………….
D- Complete the following equations:
a) NaCl + AgNo3............ + .............
b) Cu (OH)2 ............... + ..............
c) 2NaNO3 ............... + ..............
d) 2HgO ............... + ...............
E- Illustrate by experiment each of the following:
1. The importance of a catalyst in a chemical reaction.
2. The effect of the surface area on the speed of a chemical reaction.
3. The effect of temperature on the speed of a chemical reaction.
