
- •Mr. Ahmed mabrouk second term 2011
- •1(A Metal substitutes water hydrogen or acid:
- •2) The substitution of a metal instead of another one into its salt
- •1) Reaction between acid and Alkali. (Neutral)
- •2) The reaction of acid with salt
- •3 ) The Reaction of salt with salt
- •L esson 1 exercises
- •L esson 2: Speed of Chemical reactions
- •The definition of the speed of chemical reaction
- •The speed of chemical reaction:
- •Type of coherence in reactants
- •C atalysts have some common properties:
- •Biology
- •L esson 2 Exercise
- •Lesson 3: Solution
- •Solutions of acids, bases and minerals
- •Economic importance of some common acids
- •E conomic importance of some common bases
- •Economic importance of some common minerals
- •Lesson 3 Exercise
- •L esson 3 Exercise
- •Science, Technology and Society
- •A) Shinning metal
- •B) In the Kitchen
- •C) In the garden
- •D) In the medical field
- •Electromotive force ( emf )
- •T ypes of electric resistance:
- •The relationship between the current intensity and the potential difference (Ohm’s law):
- •Lesson 1 exercises
- •1 Complete the following sentences:
- •2 Choose the correct answer for each of the following statements:
- •3 Write the scientific term corresponding to each of the following statements:



Mr. Ahmed mabrouk second term 2011

Unit 1: Chemical Reactions
Lesson 1: Chemical Reactions ..................................
Lesson 2: Speed of the Chemical Reaction ............
Lesson 3: Solutions .................................................
Unit 2: Electric Energy and Radioactivity
Lesson 1: The Physical Properties of the Electric current
Lesson 2: The Electric Current and Cells ................
Lesson 3: The Radioactivity and the Nuclear energy
Unit 3: Genetics
Lesson 1: The Main Principles of Heredity .............
Lesson 2: Genes .....................................................
Unit 4: Structure and Function (Immune System and Hormones)
Lesson 1: Hormones in the Human Body ...............

 Unit
1: Lesson 1: Chemical Reactions
Unit
1: Lesson 1: Chemical Reactions
Q: What is the importance of the chemical reactions in our life?
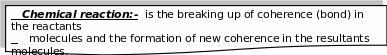
 Chemical
reactions have a great importance in our life. For
example,
Chemical
reactions have a great importance in our life. For
example,
1- When gasoline is burnt in the car engines, it generates power which makes it move.
2- Plants food is resulted from the photosynthesis process which depends on the reaction of carbon dioxide with water.
3- Different types of medicine, fertilizers and artificial filers are examples of the outcomes of some chemical reactions.

+

It
is a reaction in which  the
compound is broken up into simple substances by heat.
the
compound is broken up into simple substances by heat.
A B
A + B
B
A + B

Exp. |
Observation |
Conclusion |
1- Decomposition of metal oxides |
Red mercury oxide decomposes by heat into mercury (silver) and oxygen rises |
2 HgO 2Hg + O2 |
2- Decomposition of metal hydroxides |
Blue copper hydroxide decomposes by heat to copper oxide (black) and water vapour |
C u(OH)2 CuO + H2O |
3- Decomposition of metal carbonates |
Green copper carbonate decomposes by heat to black copper oxide and carbon dioxide. |
C uCO3 CuO + CO2 |
4- Decomposition of metal sulfates |
Blue copper sulfate decomposes by heat into black copper oxide and sulfur trioxide. |
C uSO4 CuO + SO3 |
5- Decomposition of metal nitrates |
White sodium nitrates decompose by heat into yellowish white sodium nitrites and oxygen. |
2 |

Lithium |
Li |
Potassium |
K |
Sodium |
Na |
Barium |
Ba |
Calcium |
Ca |
Magnesium |
Mg |
Aluminum |
Al |
Zinc |
Zn |
Iron |
Fe |
Tin |
Sn |
Lead |
Pb |
Hydrogen |
H |
Copper |
Cu |
Mercury |
Hg |
Silver |
Ag |
Gold |
Au |
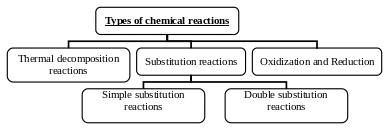
A) Simple substitution reactions
They are reactions where the element which is more active substitutes the less active one.
The series of chemical activity
It is the arrangement of metals in descending order according to their chemical activity.
A + B C A C + B
Element Compound Compound element

 NaNO3
2
NaNO2
+
O2
NaNO3
2
NaNO2
+
O2