
- •9. Mineral binders
- •9.1. Gypsum binders
- •9.3. Soluble and liquid glass, Magnesia cements
- •9.4. Hydraulic lime and lime containing binders
- •9.5. Portland cement. Technology bases
- •9.6. Properties of Portland cement. Corrosion of cement stone
- •9.7. Varieties of cements based on Portland clinker
- •9.8. Alumina cement
- •Self-Assessment Questions
Mineral binders
9. Mineral binders
Materials for bonding of heterogeneous components into artificial conglomerates of building destination (concrete, mortars, mastics, etc.) are called constructional binders. Depending on the composition they can be divided into two groups: mineral (inorganic) binders and organic binders.
Mineral binders are the powdery materials, which form a plastic mixture when mixed with water or water solutions of salts, and gradually hardens into a stony state. Depending on the conditions of hardening, the mineral binders may be categorized as non-hydraulic (air-hardening) or hydraulic.
Non-hydraulic binders can harden and gain strength only in the air conditions. Gypsum, magnesia binders, air-hardening lime and liquid glass are categorized as non-hydraulic binders.
Hydraulic binders are characterized by their ability to harden in air and in water. Portland cement, alumina cement, hydraulic lime, and alkaline-slag are some of the well-known hydraulic binders. Some binders, such as lime-silica binders, harden intensively only in autoclaving conditions.
The hydration and hydrolytic dissociation are characteristic for many mineral binders at the water or at water solutions of salts tempering. On the contrary, organic binders harden due to the coagulation structurization, condensation and polymerization reactions. For example, clays are known to harden by the coagulation mechanism.
Gypsum, limestone, clay, marl, or waste materials and by-products from different industries - slags, ashes, sludges, etc serve as the raw materials for production of mineral binders. The burning of the raw meal to pass the necessary physical-chemical processes is common working operation for most of mineral binders. They are usually ground to a powdery state to improve chemical reactivity. Different additives such as: plasticizers, hardening accelerators, micro-fillers and others are added to the mix for regulation of binding properties.
Usually, binders are used together with aggregates and dispersed or fibrous fillers are incorporated to reduce cost and to improve the technical properties of products (shrinkage reducing, increasing of the crack growth resistance, heat-resistance and others).
Most constructions works use hydraulic binders, such as, Portland and alumina cement and their modifications to produce the most widespread construction materials - cement concretes and mortars.
9.1. Gypsum binders
Materials, which consist of semi-hydrate gypsum CaSO4∙0.5H2O or anhydrite CaSO4 are called gypsum binders. They are produced as a result of the thermal treatment and grinding of raw materials such as, dehydrate or anhydrite calcium sulfate. Dehydrate - CaSO4∙2H2O is a mineral constituent of various rocks - gypsum stone, clay-containing gypsum and others, and also constituent of artificial products - industrial wastes (phosphogypsum, borogypsum etc). On heating a dehydrate, a series of chemical processes takes place, leading to the formation of substances with cementing properties. The main one being the modification of the semi-hydrate gypsum (semi-hydrate) - the product of partial dehydration of dehydrate. The basic representatives of gypsum binders are gypsum plaster and alpha gypsum(–high-strength gypsum). Both binding materials are manufactured at low-temperature burning of gypsum (120-180 ° C).
When the gypsum heating is realized in an open apparatuses - gypsum boilers or rotary kiln (Fig. 9.1) and crystallization water is selected in the form of water vapor, small crystals - CaSO4 ∙ 0.5 H2O are formed. For water of crystallization derived in the liquid state, comparatively large crystals of -semi-hydrate are formed, that is the main phase of the high strength gypsum.
T he
differences between the binding properties of -
and -semi-hydrates
can be explained by their different water demand due to the
peculiarities of crystal structure. The
he
differences between the binding properties of -
and -semi-hydrates
can be explained by their different water demand due to the
peculiarities of crystal structure. The
![]() -semi-hydrate
has higher strength due to lower water demand and consequently less
porosity in the hardened state.
-semi-hydrate
has higher strength due to lower water demand and consequently less
porosity in the hardened state.
The gypsum binders industry produces mainly gypsum plaster. The production process involves grinding and heat-treating of the gypsum stone, and fine milling of the finished product. Spread apparatus for heat treatment of gypsum plaster are gypsum frying boilers. Alpha gypsum is produced in the autoclaves. Other thermal units are also used for gypsum binders burning.
The setting and hardening of gypsum binders is conditioned by the transition of the semi-hydrate into crystalline dehydrate gypsum. The hydration and crystallization of the dehydrate gypsum runs quickly and finishes in a few minutes after tempering. The strength of gypsum increases as it dries. The main features of a gypsum plaster are: rapid setting and hardening, enhanced water demand and porosity, the ability to grow in volume up to 0.5-1% in the early-hardening period, tendency to suffer creep strain, and low water-resistance.
Q uality
of the gypsum binders is defined by the time of setting, fineness of
grinding, water demand (Fig.9.2.), compressive and bending strength.
Depending on the time of setting, gypsum binders may be divided into
such groups: rapid, normal and slow setting. The initial setting time
occur not earlier than 2 and 6 minutes, and the final set, not later
than 15 and 30 minutes respectively for the first two groups. An
initial setting time of 20 minutes or more is typical for gypsum
binders of the third group.
uality
of the gypsum binders is defined by the time of setting, fineness of
grinding, water demand (Fig.9.2.), compressive and bending strength.
Depending on the time of setting, gypsum binders may be divided into
such groups: rapid, normal and slow setting. The initial setting time
occur not earlier than 2 and 6 minutes, and the final set, not later
than 15 and 30 minutes respectively for the first two groups. An
initial setting time of 20 minutes or more is typical for gypsum
binders of the third group.
Fineness of gypsum binder grinding is evaluated by considering the amount retained on a sieve size of opening 0.2 mm.
Gypsum plaster needs up to 50-70% alpha gypsum and up to 30-40% of water for preparing a paste with normal consistency. Theoretically, 18.6% of water from the weight of binder is needed for the hydration of the semi-hydrate gypsum and all the excess water, which is removed during drying forms the voids that reduce the strength of binder. Set retarding admixtures, allow a reduction in normal consistency of up to 10-15%, and facilitate a reduction in the water demand. Adding of the superplasticizers is also effective.
It is possible to use the additives that reduce solubility of semi-hydrate at the certain concentration (ammonia, ethyl alcohol, etc.), substances that reduce the rate of crystals formation at the gypsum hydration (lignosulfonates, keratin retarder, etc.) for increasing of setting time of gypsum binders.
All gypsum
binders are graded depending on the strength. The strength of the
gypsum binders is determined by compression and bending test of a
![]() mm beams at the age of 2 hours after tempering with water.
mm beams at the age of 2 hours after tempering with water.
The gypsum binder must be protected from moisture during the transportation and storage.
Water stable gypsum-pozzolan cements is successfully used in building construction. These binders are produced by carefully mixing of 50-70% gypsum, 15-25 Portland cement and 10%-25% hydraulic admixtures. They have some advantages in comparison with the gypsum binders and Portland cement. These include: rapid hardening and strengthening, enhanced water and sulfate-resistance, plus low creep.
With respect to the scale of use, the Gypsum binder is only surpassed by cement and lime in the modern building industry. However, due to the simplicity of the technology, relatively low heat and power inputs and other advantages, their value and use is growing. Fuel requirement for production of 1 kg of the gypsum binder is 4 times lower than that used for production of 1 kg of Portland cement, and specific investments are half that for 1 kg of Portland cement.
9.2. Air-hardening lime
Product of carbonate rocks burning, which consists mainly of calcium oxide is called air-hardening lime.
T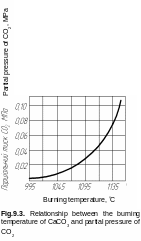 he
raw materials - chalk, limestone and dolomite should contain no more
than 6-8% of clay impurities for an air-hardening lime. The hydraulic
lime is manufactured with a greater content of impurities. Depending
on the content of active magnesium oxide, lime may be categorized as:
high-calcium (MgO <5%), magnesian (MgO = 5-20%) and dolomite (MgO
= 20-40%) and according to the fractional composition - as lump or
powdery.
he
raw materials - chalk, limestone and dolomite should contain no more
than 6-8% of clay impurities for an air-hardening lime. The hydraulic
lime is manufactured with a greater content of impurities. Depending
on the content of active magnesium oxide, lime may be categorized as:
high-calcium (MgO <5%), magnesian (MgO = 5-20%) and dolomite (MgO
= 20-40%) and according to the fractional composition - as lump or
powdery.
Types of lime include: lump quicklime, unslaked milled, hydralime and lime paste.
The lump quicklime is a semi-finished product for manufacturing of other types of lime, and it forms directly as a result of raw material burning.
The reaction of calcium carbonate dissociation CaCO3 = CaO + CO2 is a base of the process of lime manufacture. Heat of reaction is 178.16 J per 1 mole. The degree and speed of completion of the dissociation of calcium carbonate depends on the partial pressure of CO2, temperature, content of impurities, etc. Partial pressure of CO2 reaches atmospheric approximately at 900 °C (Fig. 9.3). The temperature in production terms can reach 1000-1200 ° C. The shaft and rotary furnaces mainly are used for burning of lime.
Shaft furnaces are used the most due to the simplicity of their design and operation, high thermal efficiency, relatively small capital cost of construction and high volumes of products. Shaft furnaces work continuously: through certain intervals in the upper part, limestone, anthracite or cokes are loaded, and from the lower part ready lime is unloaded. The air required for combustion comes from below and it cools the lime (Fig. 9.4).
T he
rotary furnaces are also used for burning. They have high
productivity and allow the use of small fraction of carbonate rocks
to manufacture a product which has more stable properties. The
furnaces for speed burning of lime in the boiling bed are also
effective.
he
rotary furnaces are also used for burning. They have high
productivity and allow the use of small fraction of carbonate rocks
to manufacture a product which has more stable properties. The
furnaces for speed burning of lime in the boiling bed are also
effective.
Quality of quicklime depends on the content of free active calcium oxide and magnesium oxide (activity) in it and its structure. The temperature of burning influences the reactivity of CaO and MgO. With increased temperature of burning-of limestone, the size of CaO and MgO crystals is increased. As a result of recrystallization and sintering of CaO and MgO, the lime is compacted and its reactivity decreases. The presence of particles which are slowly slaked in the lime causes internal tensions in hardenings mortars and products and results in lower strength and frost-resistance, and may lead to the appearance of cracks.
In Table 9.1 shows the requirements for high-calcium quicklime and hydrated lime. The important indicator of quality is the degree of dispersion for milled and hydrated lime, which is determined by the screenings on sieves № 02 and № 008 (opening sizes of 200 and 80 m), which must be less than 1.5 and 15%.
The active mineral additives: ash, slags and others can be added during grinding of lime to increase the water resistance and other properties of lime mortars. It is also possible to add quartz sand and gypsum.
The important feature of quicklime is the slaking ability with transition of calcium and magnesium oxides into their hydrates. This process proceeds according to the reaction: CaO+H2O = Ca(OH)2+64.9 kJ and is accompanied with the disintegration of lime pieces into fine particles. Duration of this process for a lime, which slakes quickly, lasts not more than 8 minutes, with moderate slaking rate - not more than 25 minutes and for slow rate - over 25 minutes. The process of slaking occurs quickly with high content of CaO and diminishing of burning temperature.
Таble 9.1
Requirements for high-calcium lime
Indexes |
Quicklime |
Hydrated lime |
|||
Content of active CaO+MgO, by a dry matter, (minimum %): |
|
|
|
|
|
without additives |
90 |
80 |
70 |
67 |
60 |
with additives |
65 |
55 |
– |
50 |
40 |
Content of CO2 (maximum %): |
|
|
|
|
|
without additives |
3 |
5 |
7 |
3 |
5 |
with additives |
4 |
6 |
– |
2 |
4 |
Content of the unslaked grains (maximum %) |
7 |
11 |
14 |
– |
– |
Depending on the amount of water, taken for slaking, - hydrated lime or lime paste is produced. In the first case, as a rule 0.6-0.8 parts of water are added on one part of lime by weight, and in the second case – 2-3 parts.
Hydrated lime is a finished product, which after water addition turns into lime paste. It is easier to transport and store in the packed state than quicklime. The lump quicklime and the milled lime are often slaked before the paste forming.
The lime is slaked using special devices. For small building works, the slaking of lime into the paste is made in temper boxes with plenty of water. The lime milk, formed in them is poured off to the temper pit, where the particles are fully slaked; lime is dehydrated to the consistency of the paste due to evaporation and water draining through the wooden walls of the pit in to the ground. In the temper pit lime comes through the storage at least 15 days to complete slaking and produces a plastic fine mixture. There is almost 50% of water in the lime paste, as a rule.
There are three types of lime hardening: carbonate, hydrated and hydrosilicate.
Carbonate hardening takes place in the mortars based on the slaked lime at normal temperature and pressure. Two process runs at the same time – crystallization of Ca(OH)2 from the saturated water solution and the formation of CaCO3 according to the reaction:
Ca(OH)2 + CO2 + H2O = CaCO3 +2H2O.
Crystals of the formed CaCO3 accrete with each other, with the particles of Ca(OH)2 and sand and form an artificial stone. Carbonate hardening process flows very slowly.
Hydrated hardening of lime is effected at the water tempering of the milled quicklime and takes place under certain hydration conditions (water content, heat-sink cooling). Milled quicklime dissolves in water with the formation of oversaturated solution. The effect of hardening is caused by accreting of the formed particles of calcium hydroxide.
Hydrosilicate hardening of lime occurs in lime-sand and other silicate products in conditions of high temperature and pressure of steaming, i.e., in autoclaves. The essence of it is the interaction of calcium hydroxide, silica and water and formation of new compounds - hydrosilicates that cements the sand grains. Production of lime-sand brick and silica concrete is based on the hydrosilicate hardening.
The main advantages of lime are high plasticity which gives good placeability and water-retaining ability to lime-based mortars and concretes and prevents the stratification of mixtures.
The average density of the lump quicklime is 1600-1700 kg/m3 at normal temperature of burning. It grows to 2900 kg/m3 as the temperature and duration of burning is increased. The average density of milled quicklime in the bulk state is 900-1100 kg/m3, hydrated lime is 400 -500 kg/m3, and lime paste is 1300 -1400 kg/m3.
A lime paste hardens very slowly. It is possible to strike specimens of mortars after 5-7 days. The process of setting of mortars for the milled quicklime is completed between 15-60 minutes after tempering. The lime mortars made with quicklime have large shrinkage when hardening occurs in air conditions.
Strength of materials and products based on lime and its resistance to water and to freezing and thawing depends on the type of the hardening. The highest values of these properties are exhibited by hydrosilicate and the least by the carbonate hardening. After one month hardening at normal temperature (10-20 °C), mortars based on lime paste increase their strength to 0.5-1.5 MPa and those based on the milled quicklime to 2-3 MPa. It is possible to produce lime – sand (silica) concretes with a compressive strength up to 30-40 MPa and more for the hydrosilicate hardening.
Lump quicklime is transported in boxcars in bulk or in containers. Transportation of milled quicklime and hydrated lime is carried out in paper bituminizated bags, in air-tight containers or cement trucks. It is necessary to protect the lime from moistening in the period of transportation and storage. Duration of the milled quicklime storage should not be more than 20 days, because its chemical activity quickly decreases due to interaction with atmospheric moisture.
The air-hardening lime is used for mortars which work in air dry conditions; dense and porous silicate products of the autoclave hardening; mixed hydraulic binders and lime paints.
