
6.3. Structure, composition and properties of metals
Structure of the metal alloys. Metals are typical crystalline substances properties of which are predefined by peculiarities of their crystalline structure. The certain crystalline lattice is representative for every metal. Adhesion between the elements of metal crystalline lattice is a result of physical and chemical forces action, the most substantial among which are the forces between the positively charged ions, forming the lattice, and free electrons, which gather round them (metallic bonds). The theoretical strength of metals is comparatively high and approximately equals, for example, for iron 104 MPa. However, the different metals have actual strength in oftentimes lower than theoretical one because of the defects of crystals, microcracks, different impurities and especially dislocations.
The properties of metals are usually predefined by the peculiarities of their crystallization which takes place in transition metals from the liquid state to solid. The metals are stronger and more plastic the finer the metal grains. Each metal crystallizes at strictly designated temperature. In the case of the heat removal of the melt a lot of crystallization centers appear and their intensive growing takes place. Crystallization process of metals is exothermic. The metals at a different temperature can have a different crystalline structure. These transformations which are called polymorphic, also substantially affect their properties.
Alloys as mechanical mixtures, solid solution or compounds appear at the combined crystallization of several elements.
The properties of alloys which are the mechanical mixture of accrete crystals are medium between the properties of elements, forming them.
Solid solutions and compounds can have the properties which substantially differ from the properties of elements forming them. The characteristic samples of compounds among the iron - carbon alloys are ferric carbide or cementite Fe3C, and among the alloys of aluminum copper - СuАl2.
Unlike the pure metals, crystallization of alloys is carried out not at a strictly designated temperature, but in some temperature interval between the beginning and ending of crystallization. The equilibrium state of alloys, depending on their composition and temperature, is studied in accordance with the phase diagram (Fig. 6.9). There is a row of characteristic lines and points on the phase diagram. The line of start of alloy consolidation is called the liquidus line, end of consolidation – solidus line. A point at the diagram at which the lowest temperature of alloy melting is achieved is called an eutectic.
In the iron-carbon system following basic phases form: liquid solution of carbon in the iron; ferrite that is a solid solution of carbon in - or - Fe with the body-centered cubic lattice; austenite that is a solid solution of carbon in - Fe with a face-centered cubic lattice; cementite that is ferric carbide with a rhombic lattice.
Ferrite is similar by its properties to the pure iron, it is ductile, hardness equals НВ = 80-100, elongation is 30-50 %, ultimate tensile strength – 250-300 MPa. Austenite has higher hardness and ductility than ferrite (НВ = 170-200), though the strength is lower. The cementite - one of the hardest (НВ = 800) and brittle components of iron-carbon alloys.
The solid solutions can decay (eutectoid decay) at the alloy cooling. The product of such decay of austenite at the temperature 727°C and carbon content 0.81 % is particularly pearlite - mixture of ferrite and cementite. Ledeburite - eutectic mixture of austenite and cementite forms also at the process of crystallization of iron-carbon alloys. As a result of austenite decay at a temperature 250-450°C, bainite - the superfine mixture of ferrite and ferric carbide crystallizes.
Phase transformations in the process of alloys crystallization are the basic phenomenon which is used for the change of their structure and properties. The variety of properties of steels is mainly determined by the transformations of austenite.
By the phase diagrams there are selected the alloys of such composition and structures to which are inherent the required properties.
P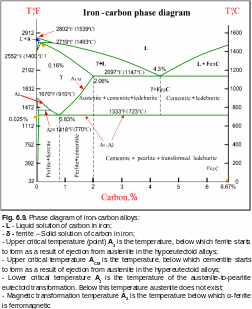 roperties
of alloys.
The choice of alloys for building
structures is mainly based on the evaluation of four main
descriptions: yield stress,
ultimate tensile strength, percent
elongation and impact elasticity at a
temperature below zero.
roperties
of alloys.
The choice of alloys for building
structures is mainly based on the evaluation of four main
descriptions: yield stress,
ultimate tensile strength, percent
elongation and impact elasticity at a
temperature below zero.
With the increasing of carbon content the amount of friable and solid cementite grows in steel, ultimate tensile strength is increased (at C < 1%) and the percent elongation diminishes. Character of influence of basic components on the steel’s properties is resulted in Table 6.3.
Steels for the metal constructions are welded well enough, if the carbon content does not exceed 0.17-0.18% in them, and total content of alloying elements is equal to 4-5%.
The low-alloy steels with the content of alloying elements to 3%, which are used in construction, have higher yield stress, less sensible to the deterioration and less capable to cold brittleness than ordinary carbon steels. The heat-resistant, corrosion-proof, wearproof and magnetic steels are the special alloyed steels.
Таble 6.3
