
- •Automotive Fuel Cell Systems
- •Ivan Surovtcev, Viktoriia Leukhina
- •Ivan Surovtcev, sPbEtu master student, I.Surovtsev.Work@gmail.Com
- •Viktoriia Leukhina, sPbEtu master student,V.Leukhina@yandex.Ru
- •Introduction
- •Fuel cell’s operation principles
- •2.1 Unit fuel cell
- •Fuel cells system
- •Fuel cells performance
- •Fuel cells applications
- •4.1 Cars
- •4.2 Buses
- •4.3 Motorcycles and bicycles
- •4.4 Railways
- •4.5 Forklifts
- •Comparison with other energy sources
Automotive Fuel Cell Systems
Ivan Surovtcev, Viktoriia Leukhina
Ivan Surovtcev, sPbEtu master student, I.Surovtsev.Work@gmail.Com
Viktoriia Leukhina, sPbEtu master student,V.Leukhina@yandex.Ru
Abstract
The article describes what is a fuel cell, internal structure of a cell and main operational principles, fuel cell performance characteristics and loses are also described. Fuel cell applications (in automotive and non-automotive systems) are listed, some examples are given. The paper also describes comparison with electric vehicle’s battery types (lead acid, NiMH and Li-on) and with conventional vehicles; benefits and disadvantages of using fuel cells are learnt. In the past, fuel cells were large and extremely expensive to manufacture, just as the first calculators and computers were. But, just like these products, the cost of fuel cells will quickly come down to consumer-affordable levels with mass production. Basic fuel cells running on pure hydrogen are pollution free, giving off only electricity, water, and heat. Economically, fuel cells represent a prudent path to provide the country's electric power because they can be installed quickly, are fuel flexible, and can be put in place incrementally, mitigating the need for more costly and sweeping changes.
Key words: fuel cell, automotive industry.
Introduction
A fuel cell is an electrochemical energy conversion device that converts hydrogen and oxygen into electricity, heat, and water. It is very much like a battery that can produce electricity while being recharged continuously. [1]
Although today's high-tech fuel cells hardly resemble their forefathers, the process upon which fuel cells are based has been known to science for more than 100 years. The first fuel cell (or "gas battery" as he called it), was invented by William Robert Grove in 1839, only 39 years after Alessandro Volto invented the battery (the voltaic cell). [1]
Fuel cell converts chemical energy in fuels into electrical energy directly, promising power generation with high efficiency and low environmental impact. Because the intermediate steps of producing heat and mechanical work typical of most conventional power generation methods are avoided, fuel cells are not limited by thermodynamic limitations of heat engines such as the Carnot efficiency. In addition, because combustion is avoided, fuel cells produce power with minimal pollutant. [2] These criteria are very attractive for automotive industry.
Fuel cell’s operation principles
2.1 Unit fuel cell
Unit cells form the core of a fuel cell.. The basic physical structure, or building block, of a fuel cell consists of an electrolyte layer in contact with an anode and a cathode on either side. Figure 1 describes this.
Schematic representation of a unit cell with the reactant/product gases and the ion conduction flow directions through the cell is shown in the figure 2, below.
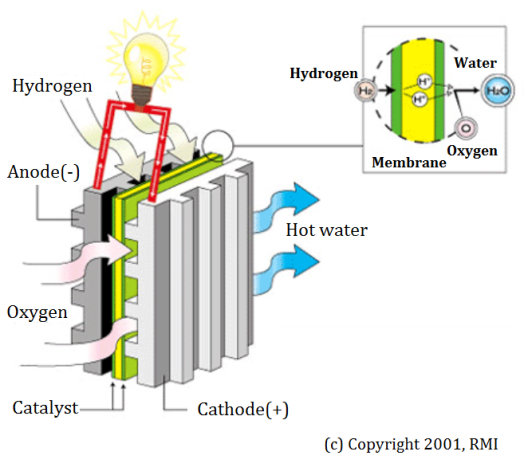
Fig. 1 - Basic structure of a unit fuel cell
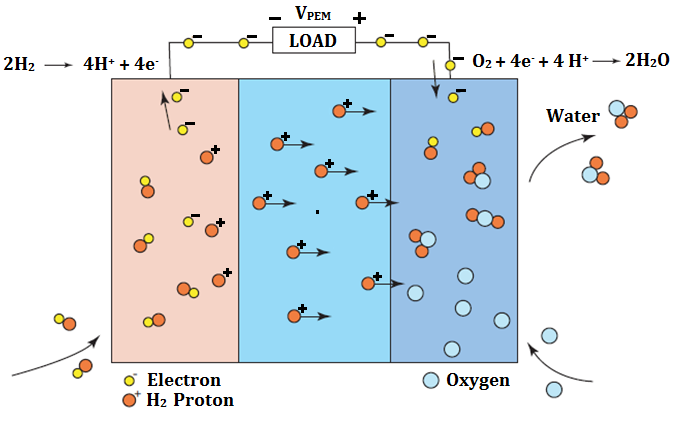
Fig. 2 – Description of internal processes
In a typical fuel cell, fuel is fed continuously to the anode (negative electrode) and an oxidant (often oxygen from air) is fed continuously to the cathode (positive electrode). The electrochemical reactions take place at the electrodes to produce an electric current through the electrolyte, while driving a complementary electric current that performs work on the load.
