
- •In this chapter we will:
- •288 Chapter 10 bacterial reproduction and growth of microorganisms
- •290 Chapter 10 bacterial reproduction and growth of microorganisms
- •Viable Count Procedures
- •294 Chapter 10 bacterial reproduction and growth of microorganisms
- •Methodology
- •Table 10-3
- •306 Chapter 10 bacterial reproduction and growth of microorganisms
294 Chapter 10 bacterial reproduction and growth of microorganisms
NEWSBREAK j
Unsafe Ice Cream Goes Undetected
An ice cream manufacturing plant in New York City in the early 1970s, in compliance with the required testing procedures to ensure the microbiological safety of its food product, routinely sent samples of the ice cream to a local quality control microbiology laboratory. The laboratory performed viable plate counts to detect coliform bacteria. The presence of conform bacteria indicates contamination with human fecal matter, making the ice cream unsafe for consumption. The plates were overgrown with coliform bacteria, and the technician at the testing laboratory recorded TNTC, the standard notation for too numerous to count. Records were compiled indicating
unsafe levels of contamination but no action was taken. This was because the Board of Health inspector who examined the records did not recognize the abbreviation TNTC and was looking for a number greater than 10 per 100 mL to signal a contamination problem. The inspector did not inquire as to the meaning of TNTC, and it was not until another inspector visited the facility and viewed the records that the problem was detected. The underlying problem with the ice cream was in the plumbing of the building, which had connected the effluent from the restrooms directly to the influent for water used in the manufacture of the ice cream.
count relies on the reproduction of individual bacterial cells to form visible colonies, which are counted to enumerate numbers of bacteria in a sample. Another problem and source of possible error associated with this technique is in the enumeration of bacteria that grow in chains or clumps that are hard to disperse. For example, a chain containing ten attached cells will grow into one colony instead of ten. Therefore using the viable plate count method to measure numbers of bacteria that tend to remain attached to one another can lead to erroneously low values.
The viable plate count procedure is selective because no one combination of incubation conditions and media allows all types of bacteria to grow.
Direct Count Procedures
Bacteria can also be enumerated by direct countinj procedures. In this procedure, counting is done with out the need to first grow the cells in culture. In om direct count procedure, dilutions of samples are ob served under a microscope, and the numbers of bac terial cells in a given volume of sample are counted These numbers are used to calculate the concentra tion of bacteria in the original sample (FIG. 10-9) Special counting chambers, such as a hemocytometa or Petroff-Hausser chamber, are sometimes employed to determine the number of bacteria. These chambers are ruled with squares of a known area and are so constructed that a film of liquid of known

FIG. 10-9 The direct counting procedure using a Petroff-Hauser counting chamber. The sample is added to a counting chamber of known volume. The slide is viewed and the number of cells determined in an area delimited by a grid. In the counting chamber shown, the entire grid has 25 large squares for a total area of 1 mm2 and a total volume of 0.02 mm3, formed by the spacing of an overlying coverslip. There are 12 cells within the single large grid (composed of 16 smaller boxes) in this example. Assuming the number of cells in this single grid is representative of all the grids, the number of cells within the total area under the grid is 12 cells. The concentration of cells is therefore 300/0.02 mm3.
ENUMERATION OF BACTERIA 295
depth can be introduced between the slide and the cover slip. Consequently, the volume of the sample overlying each square is known.
It is often desirable to stain the cells. This helps in visualizing bacterial cells. Alternatively, a known volume of a sample containing a suspension of bacteria is poured through a filter, such as a nitrocellulose 0.2 |xm pore size filter. The bacteria are stained on this filter, often using a fluorescent stain, and counted under a microscope. Many fluorescent dyes, such as acridine orange, stain all cells, making it impossible to differentiate living from dead bacteria. The difficulty in establishing the metabolic status of the observed bacteria is a major limitation of this procedure.
Bacterial cells can be enumerated by direct microscopic count procedures.
Instruments also are available for direct counting of bacterial cells. Particle counters, such as a Coulter particle counter, permit the discrimination of particles based on size so that particles the size of bacteria are counted automatically. As long as there are no nonliving interfering particles in the same size range of bacteria, this is a rapid counting method.
Most Probable Number (MPN) Procedures
Another approach to bacterial enumeration is the determination of the most probable number (MPN). MPN is a statistical method based on probability theory. In a most probable number enumeration procedure, multiple serial dilutions are performed to reach a point of extinction. The point of extinction is the dilution level at which not even a single cell is deposited into one or more multiple tubes (FIG. 10-10). A criterion is established for indicating whether a particular dilution tube contains bacteria. Such a criteria can be development of cloudiness or turbidity in a liquid growth medium or gas production (detected by its accumulation in an inverted tube). The pattern of positive and negative test results are then used to estimate the concentration of bacteria in the original sample, that is, the most probable number of bacteria, by comparing the observed pattern of results with a table of statistical probabilities for obtaining those results (Table 10-1). Often a 95% confidence limit is given in MPN tables.
The most probable number procedure is a statistical method based on probability theory used to determine bacterial populations by diluting bacterial samples to the point of extinction.
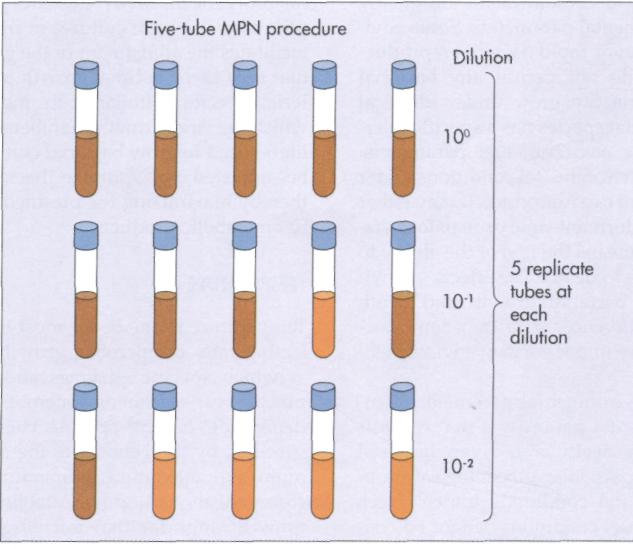
FIG. 10-10 The most probable number (MPN) procedure involves inoculation of multiple tubes with replicate samples of dilutions. The pattern of tubes that show growth (brown) and those that do not (orange) are compared with a statistical table to calculate the MPN of bacteria in the original sample. In this example all 5 tubes at the 10° show growth, 4 of 5 tubes at the 10~! dilution show growth, and only 1 of 5 tubes at the 10~2 dilution show growth. Therefore the MPN bacteria in the original sample is 170 per 100 ml (see Table 10-1).
296 CHAPTER 10 BACTERIAL REPRODUCTION AND GROWTH OF MICROORGANISMS
Ш TABLE 10-1 ^^Я

The rates of bacterial growth and death are greatly influenced by environmental parameters. Some environmental conditions favor rapid bacterial reproduction, whereas others do not permit any bacterial growth. Not all bacteria can grow under identical conditions. Each bacterial species has a specific tolerance range for specific environmental parameters. Outside the range of environmental conditions under which a given bacterium can reproduce, it may either survive in a relatively dormant state or may lose viability. Loss of viability means the loss of the ability to reproduce, which leads to death. The effects of environmental factors on bacterial growth and death rates can be seen as differences in rates of reproduction or death of a culture under varying environmental conditions.
It is a particular environmental parameter or interaction of environmental parameters that controls the rate of growth or death of a given bacterial species. Bacteria have particular physiological properties that determine the conditions under which they can grow. In nature, conditions cannot be controlled and many species co-exist; fluctuating environmental conditions favor population shifts because of the varying growth rates of individual microbial populations within the community of a given location. In the laboratory it is possible to adjust conditions to achieve optimal growth rates for a given
microorganism. Many laboratory and industrial applications use pure cultures of microorganisms. This facilitates the adjustment of the growth conditions so I that they favor optimal growth of the particular bac-1 terial species. Similarly, in industrial fermentors, I which are large growth chambers (often thousands of liters) used to grow bacterial cultures, conditions can be adjusted to optimize bacterial growth rates, thereby maximizing the production of desired bacterial metabolic products.
Temperature
Temperature is one of the most important factors af-1 fecting rates of microbial growth. The temperatures at which specific enzymes and cellular structures function varies from one microbial species to another, depending on the specific chemical compositions specified by the genome of the organism. The minimum and maximum temperatures at which a microorganism can grow establish the temperature growth range for that microorganism (FIG. 10-11), I Within this growth range there will be an optimal growth temperature at which the highest rate of reproduction occurs.
The temperature growth range is defined by the minimum and maximum temperatures at which a
X t •
Enriching for Specific Bacteria
By taking into account the physiological characteristics of specific bacterial species or types, it is possible to design conditions that favor the growth of those bacteria. This is the basis of enrichment culture technique, a method used to isolate specific groups of bacteria based on designing the culture medium and incubation conditions to preferentially support the growth of a particular bacterial type. Liquid enrichment media tend to select the bacteria that are able to grow best among all the bacteria introduced into the media. For example, to isolate bacteria capable of metabolizing petroleum hydrocarbons, one can design a culture medium containing a hydrocarbon as the sole source of carbon and energy (see Figure). By doing so, one establishes conditions whereby only bacteria that are capable of metabolizing hydrocarbons can grow. Because other bacteria cannot reproduce in this medium, hydrocarbon-utilizing bacteria are thereby selected, resulting in enrichment (increased proportions)
of the selected bacteria. Similarly, a culture medium that favors the growth of autotrophic microorganisms could be designed by providing ammonium ions and carbonate as the sole source of carbon in the medium.
The design of an enrichment procedure takes into consideration the composition of the medium and also environmental factors, such as temperature, aeration, pH, and so forth. For example, temperature can be adjusted to 5° С to favor the growth of microorganisms that live at low refrigerator temperatures or to 37° С to enrich for microorganisms that are capable of growth at the temperature of the human body. Cultures may be aerated by shaking or by bubbling with air to favor the growth of aerobes, or oxygen may be totally excluded to enrich for anaerobes. The enrichment culture technique mimics many natural situations in which the growth of a particular microbial population is favored by the chemical composition of the system and by environmental conditions.


298 CHAPTER 10 BACTERIAL REPRODUCTION AND GROWTH OF MICROORGANISMS

TABLE 10-2 ■
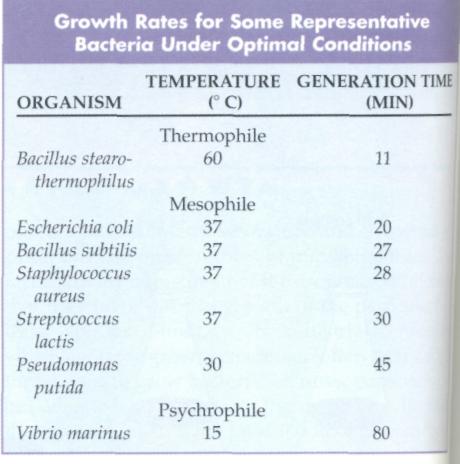
Optimal growth temperature is the temperature at which the generation time is shortest and therefore at which the maximal growth rate occurs.
Different microorganisms have different optimal growth temperatures (FIG. 10-12). Some microorgan-
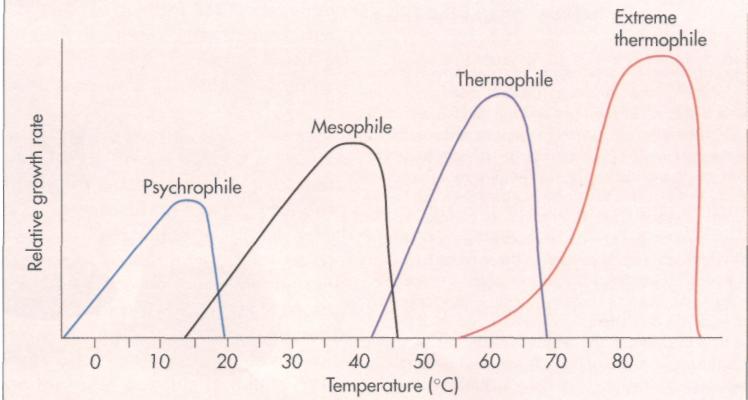
FIG. 10-12 Temperature growth ranges for mesophiles, psychrophiles, and thermophiles.
HISTORICAL PERSPECTIVE
Deep Sea Thermal Vent Bacteria
The work of Holger Jannasch established that microbial activities occur at very low rates in the deep oceans; deep ocean sediments, in effect, are biological deserts because of their low temperatures, high pressures, and low inputs of organic matter. These rates are so low that bologna sandwiches accidentally submerged inside the submersible Alvin were not decomposed during several months of exposure to microorganisms of the deep sea.
What a surprise when investigators found an area of extremely high biological productivity at a depth of 2,550 m in a region of thermal vents (subsea volcanoes) off the Galapagos Islands. The thermal vents warmed the waters, but what was the source of food supporting the growth of worms several feet long and clams several feet across? There was no light to support photosynthesis, and transport of organic matter from the surface was unlikely. The most likely explanation was that chemolithotrophic metabolism by autotrophic bacteria based on oxidation of hydrogen sulfide from the vents was providing the organic matter to support the growth of other organisms (see Figure). Establishing that bacterial chemolithotrophy was the source of organic matter would be difficult; to reach the vents in the Alvin would take hours, time on the bottom to carry out experiments would be extremely limited, and working Alvin's mechanical arms would be difficult. Nevertheless, this was the task undertaken by Holger Jannasch and Carl Wirsen of the Woods Hole Oceanographic Institution.
Jannasch and his associates were able to collect samples using specialized pressurized chambers and return living bacteria from the thermal vents to the laboratory for study. These investigators found that all surfaces intermittently exposed to H2S-containing hydrothermal fluid were covered with mats composed of layers of prokaryotic, Gram-negative cells interspersed with amorphous manganese-iron metal deposits. Enrichment cultures using thiosulfate as the energy source made from mat material resulted in isolations of different types of sulfur-oxidizing bacteria, including the ob-ligately chemolithotrophic genus Thiomicrospira. These studies established that chemolithotrophic bacteria supported the productivity of the thermal rift region.
Jannasch and other scientists then asked about the maximal temperature at which bacteria in thermal vents could grow. Bacteria were observed in waters coming from the vents with temperatures well in excess of 100° C. Could bacteria actually grow there or had the bacteria grown elsewhere at lower temperatures? What was the upper temperature limit at which bacteria can reproduce? Some scientists hypothesized that, since there was liquid water because of the high pressures, bacteria could grow at temperatures of even 500° C.
Experiments were conducted by John Baross and Jody Deming who incubated bacterial samples from the thermal vents in chambers under very high pressures at temperatures of 250° C. Because the chambers had to remain sealed under pressure to maintain the tempera-

A, Photograph of the deep sea submarine ALVIN.
300 CHAPTER 10 BACTERIAL REPRODUCTION AND GROWTH OF MICROORGANISMS
Deep Sea Thermal Vent Bacteria—cont'd

ture and prevent water from turning to steam, it was impossible to sample the chambers and culture bacteria. Deming and Baross therefore measured protein and nucleic acid content at the end of the experiment, both of which appeared to increase. Based on these observations they reported that bacterial growth occurred in the chambers incubated at 250° C. Their results were immediately questioned by many scientists. Holger Jannasch could repeatedly grow some of the bacteria from the thermal vents at temperatures of 100° to 110° C, but not at higher temperatures. No one was able to repeat the
experiments that purportedly demonstrated bacterial growth at 250° C. Independent confirmation is critical in science. Eventually it was shown by Art Yayanos at the Scripps Institute of Oceanography that the results reported by Deming and Baross could be explained by abiotic changes that occur at high temperature and pressure. Bacterial growth apparently had not occurred at 250° C. The initial report had not met the essential test of the scientific method—that of repeatability by others. The upper demonstrated growth temperature remains about 110° С
isms grow best at low temperatures. Such organisms, known as psychrophiles, have optimal growth temperatures of under 20° C. As long as liquid water is available, some psychrophilic microorganisms are capable of growing below 0° C. Psychrophilic microorganisms are commonly found in the world's oceans and are also capable of growing in a household refrigerator, where they are important agents of food spoilage.
Mesophiles are microorganisms that have optimal growth temperatures in the middle temperature range between 20° and 45° С Most of the bacteria grown in introductory microbiology laboratory courses are mesophilic. Many mesophiles have an optimal temperature of about 37° C. Many of the normal resident microorganisms of the human body, such as Eschericia coli, are mesophiles. Similarly, most human pathogens are mesophiles and thus grow rapidly and establish an infection within the human body.
Thermophilic microorganisms are organist1 with high optimal growth temperatures. The» philes, such as Bacillus stearothermophilus, growatm atively high temperatures, often growing only abova 40° C. The upper growth temperature for extern thermophilic microorganisms, such as those foundii deep thermal rift regions of the areas where volcaul activity heats the ocean water under very high pre; sure, is about 110° C. Water will remain in a liqiii state at temperatures above 100° С when it is such jected to high pressure. Thermophiles have optiis growth temperatures above 45° С and manyfe-mophilic microorganisms have optimal growth tef peratures of about 55° to 60° С One finds thai philic microorganisms in such exotic places as he springs and effluents from laundromats. Howe» many thermophiles can survive very low tempi tures, and viable thermophilic bacteria are route found in frozen antarctic soils.
FACTORS INFLUENCING BACTERIAL GROWTH 301
Psychrophiles have optimal growth temperatures of under 20° C; mesophiles grow best between 20° and 45° C; and thermophiles grow best at higher temperatures above 45° C, with 55° to 60° С often being optimal.
Oxygen
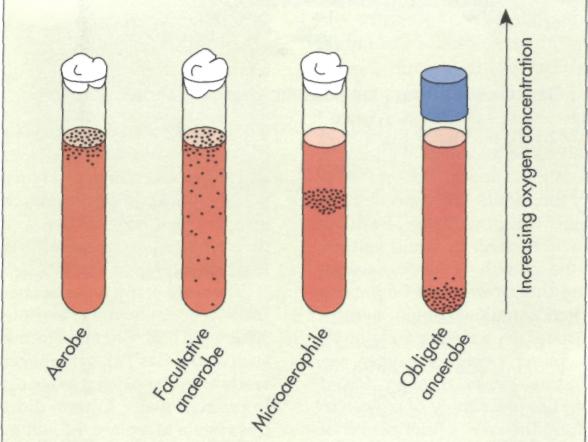
Another factor that greatly influences bacterial growth rates is the concentration of molecular oxygen. Bacteria are classified as aerobes, anaerobes, facultative anaerobes, or microaerophiles, based on their oxygen requirements and tolerances (FIG. 10-13). Aerobic bacteria (obligate aerobes) grow only when oxygen is available to support their respiratory metabolism. In laboratory cultures and industrial batch cultures, oxygen is often supplied by forced aeration or mixing (for example, on a rotary shaker) to support the growth of aerobes. Anaerobic bacteria (obligate anaerobes) grow in the absence of molecular oxygen. Anaerobic bacteria may carry out fermentation or anaerobic respiration to generate ATP. Some anaerobes have very high death rates in the presence of oxygen, and such organisms are termed strict anaerobes. Even the briefest exposure to air can kill strict anaerobes. Other obligately anaerobic bacteria, although unable to grow, have low death rates in the presence of oxygen.
While obligate anaerobes grow only in the absence of molecular oxygen, facultative anaerobes such as I coli can grow with or without oxygen. Many facultative anaerobes are capable of both fermentative and respiratory metabolism. Some are capable of both aerobic and anaerobic respiration.
Aerobes need oxygen to support their respiratory metabolism and anaerobes grow in the absence of molecular oxygen, carrying out fermentation or anaerobic respiration; facultative anaerobes grow aerobically or anaerobically.
Although oxygen is required for the growth of many microorganisms, it can also be toxic. Some microorganisms grow only over a very narrow range of oxygen concentrations. Such microorganisms are known as microaerophiles. Microaerophiles require oxygen but exhibit maximal growth rates at reduced oxygen concentrations because higher oxygen concentrations are toxic to these organisms.
Oxygen can exist in several energetic states, some of which are more toxic than others. One of these energetic states, called singlet oxygen, is a chemically reactive form that is extremely toxic to living organisms. Phospholipids in bacterial plasma membranes can be oxidized by singlet oxygen, leading to a disruption of membrane function and the death of bacterial cells. Peroxidases in saliva and phagocyte cells (blood cells involved in the defense mechanism of the human body against invading microorganisms) generate singlet oxygen, accounting in part for the antibacterial activity of saliva and the ability of phagocytic blood cells to kill invading microorganisms.
Singlet oxygen is chemically reactive and extremely toxic to living organisms.
The conversion of oxygen to water occurs when oxygen serves as a terminal electron acceptor in respiration pathways. This involves the formation of an
