
- •Экстракция тестикулярных сперматозоидов (tese): выделение, криоконсервация, икси
- •Хирургическое извлечение и подготовка тестикулярной ткани для икси и криоконсервации
- •Обструктивная азооспермия.
- •Необструктивная азооспермия
- •Работа с тестикулярной тканью (Извлечение тестикулярных сперматозоидов)
- •Приготовление гомогенной тестикулярной суспензии
- •Механическое выделение тестикулярных сперматозоидов
- •Криоконсервация
- •Размораживание
- •Икси с использованием тестикулярных сперматозоидов
- •Реакция сперматозоидов на hos-тест
- •Лазерный тест на витальность сперматозоидов (a неживой, b и c живой)
- •Повторное замораживание
Markus Montag
Testicular sperm extraction (TESE):
Sperm preparation, cryopreservation, ICSI
Coarse Manual
Маркус Монтаг
Экстракция тестикулярных сперматозоидов (tese): выделение, криоконсервация, икси
руководство к практикуму
________________________________
30-31 October 2004, Moscow
30-31 октября 2004, Москва
Surgical removal and preparation of testicular tissue for ICSI and cryopreservation
The first step prior to a testicular biopsy is a careful and extended andrological work up of the male patient. In addition, all patients who are finally treated by TESE should be informed, that the aneuploidy rate in embryos derived from ICSI with testicular spermatozoa is significantly higher (Bernardini et al., 2000; Silber et al., 2003). The patients should be also informed, that in some patients the reason for being infertile can be attributed to deletions in the Y chromosome and that consequently a boy derived by TESE-ICSI will have the same genetic defects like his father and has a high chance of being also infertile if he is grown up (Silber & Repping, 2002).
If a patient´s spermiogramm analysis reveals an azoospermia, one should confirm the azoospermia by at lest two more sperm analysis on different occasions in order to verify the diagnosis. It is also extremely important, that not only a conventional sperm analysis is done, but that the whole ejaculate is washed and centrifuged and that the pellet or sediment is resuspended and screened for the presence of spermatozoa (Ron-El et al., 1997). During sperm preparation for this diagnostic check it is mandatatory to wash and pellet the whole ejaculate. It should be avoided to perform a sperm preparation with a gradient (e.g. Percoll), as within a gradient preparation the loss of spermatozoa is very high. Usually it is helpful to prepare a dish with at least 3-4 droplets of culture medium (50 – 100 µl each) which are covered by mineral oil. In each droplet 5 – 10 µl of the resuspended sediment from the ejaculate are placed and the dish is placed into an incubator for 30 minutes. After this incubation period, the dish is removed from the incubator and placed on an inverted mircroscope, preferentially the one used for ICSI. One should screen very carefully at the border of the droplet with a 20x objective for the presence of spermatozoa. If no spermatozoa are at the border, one should also screen within the droplets in order to search for immotile spermatozoa. If no spermatozoa are found in at least two ejaculates using this diagnostic assay, one can consider that the patient truly has an azoospermia.
Dish for screening spermatozoa in a patient with suspected azoospermia for TESE

50 -100 µl Hepes-buffered-medium
covered with mineral oil
If the patient is diagnosed as being azoospermic and shows in addition signs of a genital inflammation (e.g. high number of leukocytes in the ejaculate), one should consider an anti-inflammatory and antibiotic treatment with Diclofenac and Doxycycline for 3-4 weeks prior to another sperm analysis. Sometimes it is possible, that a few spermatozoa are present after such a treatment despite the diagnosis of an azoospermia during the first examinations (Montag et al., 1999; a PDF file of this article can be found on the CD).
Once the patient is diagnosed as truly azoospermic, one should try to find out the reason for the azoospermia, in other words: if it is an obstructive or a non-obstructive azoospermia. This decision is already important for the final treatment, because patients with an obstructive azoospermia usually do present with spermatozoa either in the epidydymis or in the testis. Under such circumstances it is possible to perform the biopsy of the male at the same day like the follicular puncture of the woman.
However, if there is a doubt about the presence of spermatozoa in the testis, like it is very often the case for non-obstructive cases of azoospermia, one may consider to perform the testicular biopsy before the stimulation of the woman is started. If spermatozoa are present in the biopsied material, one can cryopreserve them and start the ART cycle. If no sperm are present, one can discuss with the patients and probably even recommend donor sperm. This strategy will help to avoid a costly and ineffective stimulation of the woman.
Obstructive azoospermia
In patients with an obstructive azoospermia one should consider two things:
these patients have a higher frequency for a mutation in the gene for cystic fibrosis. Because mutations in this gene have in general the highest frequency among Europeans, one must not only screen for that mutation in the male, but also his spouse. If both are carrier of the mutation, the children are at a 50% risk to have mucoviscidosis and if the physician did not counsel these patients properly, they may sue him for false treatment
In cases of obstructive azoopsermia, the sperm production in the testis is usually not disturbed, meaning that spermatozoa are produced in the testis, transported to the epididymis and get destroyed within the epididymis. However, it is possible to retrieve spermatozoa from the epididymis by the so-called microsurgical epididymal sperm aspiration (MESA). Only if these spermatozoa are motile, it is worth while to use them for ICSI or for cryopreservation. Therefore we recommend that the surgeon or a reproductive biologist should check immediately after the MESA in the surgical theatre if motile spermatozoa are present in the aspirate. If not the surgeon has to proceed and do a testicular biopsy in order to get spermatozoa which are able to fertilize.
The MESA surgery is more time-consuming than a testicular biopsy. It must be performed by an experienced urologist, as the epidydimis must be prepared in a way which allows direct access to the caput and cauda. Prior to the surgery, the surgical department must inform the reproductive laboratory at which day and at what time the surgery is scheduled. The reproductive laboratory should deliver to the surgical team sterile, HEPES-buffered culture medium as well as sterile tubes (at least 2 ml, e.g. cryotubes). The surgical team must make sure, that the sterile HEPES-buffered culture medium is pre-warmed to 37°C prior to the surgery and that it is kept in or close to the surgical theatre.
As mentioned above, it is extremely important that the quality of the MESA aspirate is checked and that the dialogue between the surgical team and the reproductive biology team is optioned. The aspirate should immediately be placed into the warm medium and must be kept warm during the transport to the reproductive laboratory.Usually one can retrieve by one MESA procedure enough motile spermatozoa for 6-8 probes for cryopreseravtion, equivalent to 6-8 ICSI treatment cycles. MESA probes are much easier to handle by the reproductive laboratory, because the preparation only needs to be washed once with culture medium before being processed for cryopreservation.
Non-obstructive azoospermia
In cases of non-obstructive azoospermia, the only chance to retrieve spermatozoa is directly from the testis. However, these cases are usually more difficult than patients with a non-obstructive azoospermia, because the etiology of the underlying azoospermia is not known and therefore it is difficult to predict if spermatozoa will be found during the biopsy or not.
In order to give the patient a prognosis one cann assess serum FSH levels. However, even in patients with high FSH levels, spermatozoa can be present in the biopsy material. Therefore the value of FSH as a prognostic factor is unclear. The same holds true for screening of deletions in the AZF region of the Y chromosome in these pateints. Again there is not yet a common sense about that criterion.
The only factor which seems to be a good prognostic factor is serum inhibin B in the azoospermic male patient (Ballesca et al., 2000; article on the CD). The inhibin B concentration is significantly lower in men in whom TESE failed (<15 pg/mL), although in these males the FSH concentration did not differ. Therefore one should inform patients with an undetectable inhibin B concentration about the low chances of positive testicular biopsy.
Spermatozoa recovery shows no correlation with testicular volume, however, there seems to be a good correlation between successful testicular sperm recovery and testis histopathology. Therefore testicular histopathology can be used as a predictor of successful sperm recovery and a histopathology should be always done with a small piece of the testicular tissue which was removed from the testis.
In general, the removal of testicular tissue from the testis is a surgical procedure which should be done by an experienced urologist or andrologist. However, like with MESA, it is extremely important, that the surgical team and the reproductive laboratory do communicate with each other before the surgery and during / after the surgery.
Prior to the surgery, the surgical department must inform the reproductive laboratory at which day and at what time the surgery is scheduled. The reproductive laboratory should deliver to the surgical team sterile, HEPES-buffered culture medium as well as sterile tubes (at least 2 ml, e.g. cryotubes). The surgical team must make sure, that the sterile HEPES-buffered culture medium is pre-warmed to 37°C prior to the surgery and that it is kept in or close to the surgical theatre.
The strategy during the surgery depends on the situs of the testis and on the knowledge from the inhibin B levels. If the patient has both testis and if both are large enough, more material can be retrieved compared to the situation when only one small testis is present. Similar, if inhibin B level is low, more tissue needs to be excised compared to normal inhibin B levels.
The removal of testicular material causes a depletion of cells producing testosterone and if too much testicular tissue is taken away, the patient will require hormone substitution with testosterone for the rest of his life. This means that the surgical team has to decide during the surgery how to go on.
If the anamnesis gives any clue to a testicular spermatogenetic arrest with a testicular damage or if inhibin B levels are low, when the biopsy should be made at multiple loci, as in such cases if at all only a focal spermatogenesis exists and usually the islands producing spermatozoa are inside the testis and not at the testicular periphery.
It is advisable to do the surgical procedure as an open biopsy and with a surgical microscope (Kamal et al., 2004). The benefit is that one can avoid the damage of blood vessels during surgery – which is beneficial for the patient´s health and for the recovery of the testis (Schlegel, 1999, article on the CD). In addition, in cases of extremely poor spermatogenesis – like e.g. in Klinefelter patients – this method allows to identify under the surgical microscope those few tubules which are swollen and these are the ones which usually still produce spermatozoa and should be removed.
Following the surgical excision of the testicular material, the biopsy material must be placed immediately into the pre-warmed HEPES-buffered medium in the sterile tube, where the pieces from the left or right testis are put into separate tubes and the site of removal should be noted on the tubes. Until to the arrival of the material in the reproductive laboratory, the material must be kept warm.
It is extremely important that a small part of testicular tissue is removed for pathology. This should be placed e.g. into Bouin´s fixative and given to a pathologist with the request to perform a pathological as well as histological diagnosis. The underlying reason is, that male azoospermic patients have a higher incidence for a carcinoma in-situ which can be only detected by this way. Secondly, the histological examination can be compared to the outcome of the preparation in the reproductive laboratory and the combined information can be used to give the patient a prognosis for this treatment cycle, as well as for the possibility to perform another biopsy in the future.
Wetprep
Within the surgical theatre and immediately after the excision of the testicular material, an experienced urologist or a reproductive biologist should perform a so-called wetprep. Therefore a small piece of the excised tissue is taken onto a slide and a cover slip is pressed on top of the tissue, so that the tubules within the tissue piece do rupture. Using a phase contrast microscope with a 20x / 40x objective could give a first idea about the presence of spermatozoa. However, if no spermatozoa are found, there is still the possibility that the more extensive preparation in the reproductive laboratory can reveal some spermatozoa.
Transfer of the tissue to the reproductive laboratory
After surgery, the material must be constantly kept warm (37°C). The surgical team should inform the reproductive laboratory as soon as possible and the probes must be transferred from the surgical place to the reproductive laboratory. Also during this transport the tissue must be kept warm.
Procedure of tissue preparation
At present there are two protocols which are used for testicular tissue preparation and the principles are explained shortly.
Protocol 1: Block freezing and preparation after thawing
The block freezing method is used because some authors claim that the block freezing gives a better cryo-survival of the testicular spermatozoa (Salzbrunn et al., 1996). However, the group in Bruxelles has shown in a study, that in the direct comparison, freezing of a homogenate was superior to block freezing (Crabbé et al., 1999).
Another great disadvantage of the block freezing method is the phenomenon of the focal spermatogenesis. It could be, that one block with testicular tissue may contain some spermatogenetically active tubules, while another block does not. This means that the reproductive laboratory will only know at the day of thawing and preparation, if spermatozoa will be available for ICSI or not.
Protocol 2: Preparation prior freezing and freezing of a homogenous solution
Prior to the take over of the Bonn IVF laboratory by Dr. Montag, this lab did the block freezing method. Dr. Montag changed this strategy and introduced the strategy of freezing a homogenate. The first reason is, that we know after the preparation and therefore prior to the freezing, how the quality of the preparation is and if we have enough spermatozoa for several ICSI cycles or not. Because we prepare a homogenate from the complete testicular material, we do have no problems with focal spermatogenesis and the quality of aliquot No.1 should be identical to that of aliquot No.2, No. 3, etc. Secondly, the results from the Bonn cryo-TESE programme improved immediately with changing from the block freezing method to the homogenate freezing method. This may be an indicator, that spermatozoa frozen in suspension do possess better fertilization potential compared to spermatozoa frozen in tissue blocks.
Protocols for making a homogenous testicular suspension
Again there exist two protocols for generating a homogenous testicular suspension: the mechanical method and the enzymatic method.
The enzymatic method uses chimotrypsin and trypsin inhibitor, and the testicular blocks are incubated in an enzymatic solution for up to 6 hours. Therefore this method is time-consuming.
!This method will be not part of the workshop, but if one is interested, one can read about this method in these articles: Salzbrunn et al., 1996.
The mechanical method, which is described in detail on the following pages, has the advantage that it can be performed within minutes. A large study in Germany revealed, that the success rate of TESE-ICSI cycles was similar when the testicular tissue was prepared by enzymatic or by mechanic way (Baukloh, 2002 – article on the CD). Therefore we favour the mechanic way because the results are obtained within 30 minutes.
Mechanic preparation of testicular spermatozoa
The warming plate must be kept at 37°C during the preparation and should be placed within a sterile cabinet or lamina air flow.
A sterile slide is placed into the large sized dish (e.g. Falcon 1001) and is placed onto the warming plate. The testicular tissue is removed from the tube and is placed inside the dish together with 3-4 ml of HEPES buffered medium. To get a better diagnosis and correlation with the pathological / histological examination, the tissue from each side should be prepared separately. Next, the testicular tissue is moved onto the slide inside the dish, so that during cutting of the tissue no plastic material is cut out of the dish as this may contaminate the tissue.
Dish
with slide, human tissue, scalpels and slides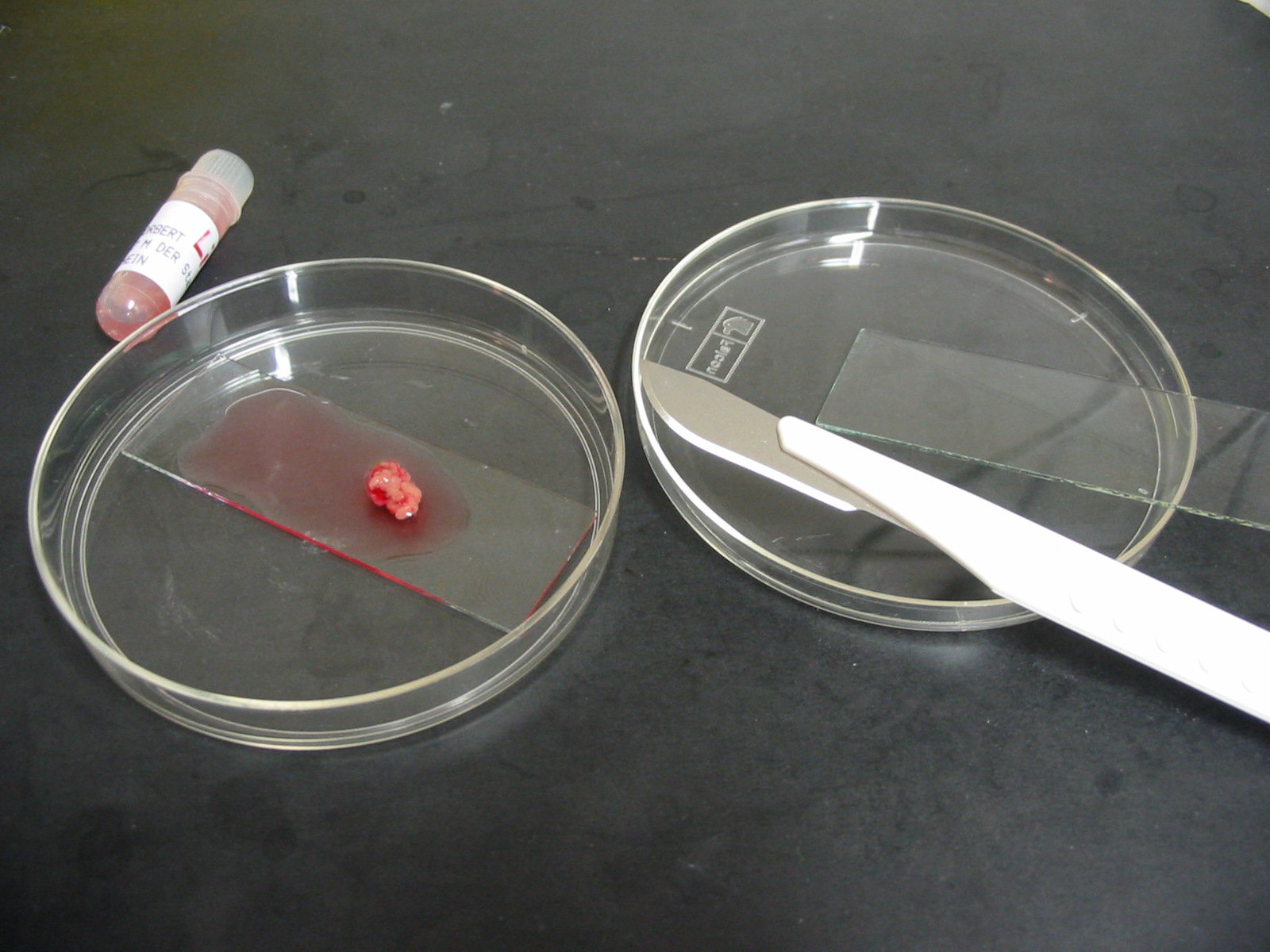
There are two methods to cut the testicular tissue into small pieces:
Using two scalpels, the testicular pieces can be cut into small pieces – as small as possible (cube 1-2 mm x 1-2 mm x 1-2 mm), or
Using one scalpel and another sterile slide, or even two sterile slides, one can place the edges of the slides side by side on the tissue, press the tissue to the bottom and then move both slides away from each other, but still pressing the slides to the bottom. This method will squeeze the spermatozoa directly out from the tubules.
Suspension after dissection
with scalpel and slide
Eppendorf tube and pistil inside
O nce
the testicular tissue is fine enough, the tissue is transferred with
a 1 ml syringe or another pipette with a wide opening into the 1.5 ml
or 2.0 ml reaction tube (preferentially Eppendorf style). The tissue
should be allowed to settle to the bottom of the tube and excess
medium must be withdrawn into a 5 ml tube. The special Eppendorf
pistille is introduced into the tube and the tissue is carefully
minced by moving the pistille slowly up and down while turning at the
same time.
nce
the testicular tissue is fine enough, the tissue is transferred with
a 1 ml syringe or another pipette with a wide opening into the 1.5 ml
or 2.0 ml reaction tube (preferentially Eppendorf style). The tissue
should be allowed to settle to the bottom of the tube and excess
medium must be withdrawn into a 5 ml tube. The special Eppendorf
pistille is introduced into the tube and the tissue is carefully
minced by moving the pistille slowly up and down while turning at the
same time.
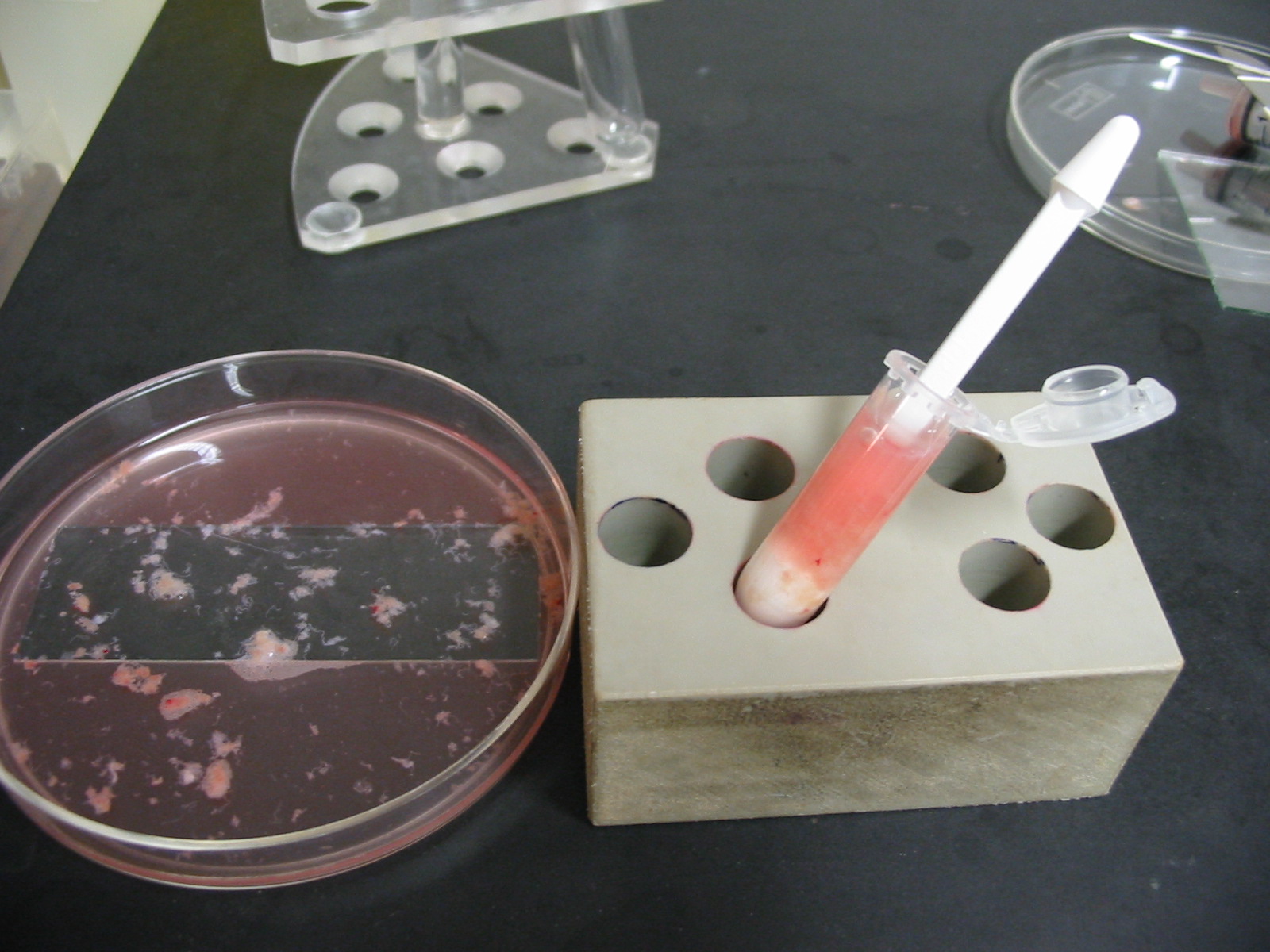
If the tissue is nearly homogenous, the homogenate should be removed and put into the 5 ml tube where already the medium from the step before is located. This step must be repeated with all small tissue pieces which are still in the large dish. Once all small tissue pieces have been minced with the pistille and are in the 5 ml tube, a long Gauge syringe (90 mm) is connected to a 5 ml syringe and the complete testicular tissue homogenate is roughly pipetted through this syringe in order to rupture some remaining tubules and tissue pieces.
If still some larger tissue pieces are present in the 5 ml tube, then the homogenate must be carefully aspirated and transferred into a fresh 5 ml tube without these pieces (which can be frozen separately or discarded).
Prior to the next step, 10 µl are removed from that homogenate and are placed on a slide, covered with a cover slip for examination under a normal microscope equipped with a 40x phase contrast objective. The entire droplet must be screened carefully for the presence of spermatozoa, or elongated spermatozoa or round spermatids. The concentration of spermatozoa will give an orientation of how many aliquots can be frozen for future ICSI cycles. Usually one will not see motile sperm, as sperm motility only is present after a longer incubation in culture medium. If approximately 10 spermatozoa are seen on the whole slide, one can judge that approximately 100 – 300 µl are enough for one ICSI therapy using the TESE spermatozoa. From this estimate one can determine the maximum number of aliquots for freezing.
Following the microscopic examination, the homogenate is centrifuged for 5 minutes at approximately 600 – 900 g.
If no sperm were present in the first examination, the pellet should be resuspended in 100 µl of HEPES-buffered medium and 5 µl should be used for another microscopic examination in a droplet of 50 µl medium covered with mineral oil and using the inverted ICSI microscope (as described in the introductory section for diagnosis of azoospermia). If again no spermatozoa are found, one should consider freezing only one aliquot until the pathologist has made the histological diagnosis of the testicular situation in toto.
If spermatozoa were spotted in the first examination, the pellet is resuspended in 0.5 – 1.5 ml, depending on the size of the pellet and the sperm concentration using a HEPES-buffered culture medium.
Cryopreservation
In our clinics we usually make a maximum of 4-5 aliquots for cryopreservation because our experience has shown that the TESE patients do not perform more than 4 treatment cycles. Therefore, if we have enough spermatozoa, we resuspend the pellet in 1.5 ml HEPES-buffered culture medium and we add 1.05 ml of sperm freeze solution (70% volume of 1.5 ml = 1.050 ml; these values depend on the freezing medium used in the laboratory and may differ with other media) which was kept at least for 30 minutes at room temperature to the resuspended pellet. It is extremly important, that the sperm freeze medium is added slowly and drop by drop, otherwise the spermatozoa will suffer from an osmotic shock reaction and will loose their ability to regain motility in the medium after thawing. The total amount of 2.5 ml is aliquoted into 4-5 cryotubes, with 0.5 ml in each tube. Prior to starting the freezing process, the tube containing the freezing mixture above should be allowed to equilibrate for 15 minutes.
The freezing process itself can be performed using a freezing machine or simply by placing the cryotube (we use 2 ml Nunc cyro tubes) approximately 5-8 cm above the upper level of liquid nitrogen in a container for approximately 30 minutes prior to direct plunging into the nitrogen. Like all cryopreserved materials, testicular tissue can be stored for a very long time without loosing its potential for being used in an ICSI cycle.
Thawing
For thawing, one tube is removed from the cryotank and immediately placed into warm water (37°C) to warm the solution. The medium (0.5 ml) is transferred into another 1.5 ml centrifugation tube and 1 ml of warm HEPES-buffered medium is added and mixed. After centrifugation for 2-3 minutes at 4000 rpm (2000g), the supernatant is discarded and the pellet with the sperm is resuspended in 20 – 40 µl with medium. Immediately after resuspension, 5 µl aliquots must be placed into the injection dishes (see below).
ICSI using testicular spermatozoa
For one patient one should prepare 1 - 2 injection dishes as shown in the corresponding Figure:
Dish for injection in a TESE-ICSI case

 3-5
µl 10% PVP
3-5
µl 10% PVP

 3-5
µl HEPES-buffered-medium / one droplet per oocyte
3-5
µl HEPES-buffered-medium / one droplet per oocyte
50 µl HEPES-buffered-medium + 5-10 µl suspension
covered with mineral oil
It is important that not too much of the testicular suspension is placed into one droplet. It is a good strategy to pipette 10 µl of the suspension into the droplet no. 1 on top, followed by up and down pipetting to mix droplet 1. Then 10 µl from droplet 1 are removed and pipetted into droplet 2, again with mixing. Following this, one should check the concentration in droplet 1 and droplet 2 under the inverted microscope.
If the mixture is too dense in droplet 2, one can again take 10 µl from droplet 2 and mix this into droplet 3.
If the mixture is good in droplet 2, one can take another 10 µl from droplet 1 and mix that into droplet 3.
It is advisable to remove prior to screening for spermatozoa all large tissue remnants which are still in the droplet, because if not, these will stick to the capillary and lead to a clotting of the capillary.
Before the isolation of spermatozoa, one has to decide on how to isolate the sperm cells from the droplets with the testicular suspension. If the concentration and thus the total number of spermatozoa is very low, one must use a very high density of material within one droplet in order to be able to find spermatozoa. Due to a high density it can be very difficult to isolate the spermatozoa by using a normal ICSI capillary, as this capillary may get blocked by the large amount of cellular debris in the droplet and this makes it impossible to isolate spermatozoa efficiently. In such a case we prefer the use of a wide-opened capillary for isolation of spermatozoa from the droplet and for transfer into either a medium droplet or into PVP. A good choice is a capillary which is intended to be used for polar body biopsy and has an inner diameter of approximately 16-20 µm.
If the concentration of spermatozoa is good and if sperm cells can be retrieved easily without having too much cellular debris around, one can immediately use the ICSI capillary to withdraw the spermatozoa from the droplet. It is advisable to suck the spermatozoa into the capillary head first, as this goes much easier and without getting cellular debris. Once inside, the capillary is carefully removed from the droplet and the sperm cell is released either in a small droplet of culture medium (if the injection takes place after more than 30 minutes) or directly into PVP. The most important step is, that the capillary is lowered into the droplet from the top and only at the moment when the sperm cell should be picked up.
The primary criteria for the selection of spermatozoa is motility. Usually the spermatozoa settle very fast at the bottom of the dish and one can screen for spermatozoa already after 10 minutes. However, following a prolonged incubation for approximately 1-2 hours, the spermatozoa undergo a maturation process within the culture medium and some may acquire motility. Therefore it is advisable to prepare the dish and load the droplets 2 hours prior to ICSI. Usually one will not see spermatozoa with a progressive motility, instead the spermatozoa will show a very subtle motility, mostly by some curving of the sperm tail. In order to see this gentle movement, one should take the time to observe an individual spermatozoa for 10 – 20 seconds in order to see if any bending or curving of the sperm tail is apparent.
!Cave: In the testicular preparation one can see the so-called Brownian movement, which is a movement caused by molecular force interactions. This movement is also active on spermatozoa, however, such a pseudo-motility is different from the real motility, because the real motile sperm show e.g. bending of the sperm tail, whereas the false motile sperm are moving as a whole!
If there are no motile spermatozoa at all, one can either choose the spermatozoa by random or one can apply a test to check for sperm viability. There are two test methods described in the literature:
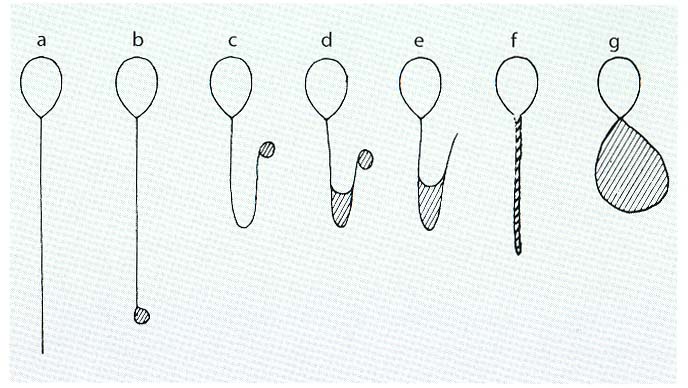
Method 1 uses the so-called hypo-osmotic swelling test (HOS-Test; Jeyendran et al., 1984). This method only works with fresh TESE and not with cryo-TESE probes, which is a big disadvantage of this method. Spermatozoa which show HOS reactivity of type b-g (see Figure below) can be used for injection and will give higher fertilization rates compared to spermatozoa of type a.
Method 2 uses a newly described technique which is based on the fact that a laser shot to the tail of a viable sperm will cause a reaction which is similar to the HOS-reactivity. This method also works with cryopreserved – thawed testicular spermatozoa (Aktan et al., 2004).
HOS reactivity of spermatozoa in the HOS medium
Viability test using a laser system (A non-viable, B/C viable)
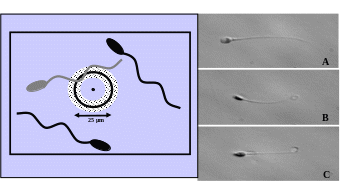
Although testicular spermatozoa do not move very much, it is extremely important to perform a very aggressive sperm immobilisation process just prior to injection. It has been shown, that this aggressive treatment is especially helpful in the case of testicular spermatozoa in order to increase the fertilization and pregnancy rates (Palermo et al. 1999).
If no spermatozoa are present, one can inject elongated spermatids or round spermatids. Elongated spermatids can easily be identified because these cells look like sperm cells except that the sperm tail is absent and a cytoplasmic sac is present. The identification of round spermatids is extremely difficult and to date there are only few pregnancies and live births following injection of round spermatids.
The problem with injection of elongated spermatids as well as with the injection of only immotile sperm is, that the oocyte may not get activated due to an impaired activation potential of these sperm cells. Therefore one can try to support oocyte activation by the use of calcium ionophore, which means that after ICSI the oocytes are immediately incubated in culture medium containing calcium ionophore (5-20 µM) for 15 – 20 minutes, washed carefully in 5 droplets with normal culture medium before being incubated in culture medium.
Re-freezing
In some individual cases, where we had only one portion of cryopreserved testicular material from an outside clinic, we did thaw the material and we used part of it for TESE-ICSI, whereas the other part was again processed for cryopreservation. Although this is not a method which should become general standard, this procedure works well and we even have two pregnancies from such re-frozen material.
Pronuclear formation
With some patients one can observe that the formation of pronuclei takes longer than usual. This can be attributed to the testicular sperm of that patient, which were probably not absoloutely mature. Although we do transfer the embryos derived from such cycles, the prognosis / pregnancy rate is not good.
Equipment and material for testicular tissue preparation
1 inverted microscope for ICSI with 20x or 40x objective (Hofmann or Phase contrast) and a camera with monitor for demonstration
1 normal microscope with 40x Phase contrast objective
1 warming plate (37°C)
Centrifuge for 10 – 15 ml tubes
Centrifuge for 1.5 – 2 ml tubes (or suitable adapter for the centrifuge above)
Slides (also if possible: sterile slides, packed by 2 / package and heat sterilized)
Cover slips
5 ml plastic Syringes
Needles for syringe (20G x 1½, 0.9 x 40 mm and 20G x 2¾, 0.9 x 70 mm)
Dishes with 10-15 cm diameter
Scalpels fig. 22, fig. 11
HEPES-buffered medium (37°C) for preparation
Centrifugation tubes (10 – 15 ml)
Centrifuge tubes 1.5 – 2 ml (preferentially Eppendorf)
Cryotubes (Nunc)
Marker to write on cryotubes
Medium for cryopreservation (10 – 20 ml, sperm cryo medium, glycerol based)
Microistille for Eppendorf tubes (order number 0030 120.973
Pipettes for small volume (10 – 200 µl)
Pipette tips for pipettes above
Facilities for cryopreservation and long-term storage of cryopreserved testicular tissue
Appendix
On the CD several Windows Media Files are included. For further details please open the Word document Read me first on the CD. There are also some PDF files with relevant literature included on the CD.
References
Ballesca JL, Balasch J, Calafell JM, Alvarez R, Fabregues F, de Osaba MJ, Ascaso C, Vanrell JA.: Serum inhibin B determination is predictive of successful testicular sperm extraction in men with non-obstructive azoospermia. Hum Reprod. 15, 1734-1738 (2000). On CD
Baukloh V.: Retrospective multicenter study on mechanical and enzymatic preparation of fresh and cryopreserved testicular biopsies. Hum Reprod 17, 1788-1794 (2002). On CD
Bernardini L, Gianaroli L, Fortini D, Conte N, Magli C, Cavani S, Gaggero G, Tindiglia C, Ragni N, Venturini PL.: Frequency of hyper-, hypohaploidy and diploidy in ejaculate, epididymal and testicular germ cells of infertile patients. Hum Reprod. 15, 2165-2172 (2000). On CD
Crabbé E., Verheyen G., Tournaye H., Van Steirteghem A.: Freezing of testicular tissue as minced suspension preserves sperm quality better than whole-biopsy freezing when glycerol is used as cryoprotectant. Int J Andrology 22, 43-49 (1999).
Jeyendran RS, Van der Ven HH, Perez-Pelaez M, Crabo BG, Zaneveld LJD (1984) Development of an assay to assess the functional integrity of human sperm mebrane and its relationship to other semen characteristics. J Reprod Fertil 70:219-228.
Aktan T. M., Montag M., Duman S., Gorkemli H., Rink K., Yurdakul T. (2004) The use of a laser to detect viable but immotile spermatozoa. Andrologia 36, 1-4.
Kamal A, Fahmy I, Mansour RT, Abou-Setta AM, Serour GI, Aboulghar MA.: Selection of individual testicular tubules from biopsied testicular tissue with a stereomicroscope improves sperm retrieval rate. J Androl. 25, 123-127 (2004).
Montag M., van der Ven H. and Haidl, G.: Recovery of ejaculated spermatozoa for intracytoplasmic sperm injection in an azoospermic patient with genital tract infection. Andrologia 31, 179-181 (1999). ON CD
Palermo G.D., Schlegel P.N., Colombero L.T., Zaninovic N., Moy F., Rosenwaks Z.: Aggressive sperm immobilization prior to intracytoplasmic sperm injection with immature spermatozoa improves fertilization and pregnancy rates. Hum Reprod. 11, 1023-1029 (1996).
Ron-El R, Strassburger D, Friedler S, Komarovski D, Bern O, Soffer Y, Raziel A.: Extended sperm preparation: an alternative to testicular sperm extraction in non-obstructive azoospermia. Hum Reprod. 12, 1222-1226 (1997).
Salzbrunn A., Benson DM, Holstein A.F., Schulze W.: A new concept fort he extraction of testicular spermatozoa as a tool for assisted fertilization (ICSI) Hum Reprod 11, 752-755 (1996).
Schlegel PN.: Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Hum Reprod.14, 131-135 (1999). ON CD
Silber S, Escudero T, Lenahan K, Abdelhadi I, Kilani Z, Munne S.: Chromosomal abnormalities in embryos derived from testicular sperm extraction. Fertil Steril. 79, 30-38 (2003).
Silber SJ, Repping S.: Transmission of male infertility to future generations: lessons from the Y chromosome. Hum Reprod Update. 8, 217-229 (2002).
