
- •The Basic equations of molecular-kinetic theory of Gases.
- •Temperature.
- •Dependence of pressure on the molecules concentration and temperature.
- •Boltsman's constant.
- •Avogadros number, molar mass.
- •Ideal gas and its properties.
- •Ideal gas state equation.
- •Molar mass.
- •Gas constant.
- •Internal energy of ideal gas. Change of internal energy.
- •Heat, specific heat, molar heat.
- •Barometrical formula.
- •Boltsman's distribution.
- •Internal friction.
- •Heat conduction.
- •The first thermodynamics law. Its application for Isoprocess. Gas work at constant pressure and constant temperature.
- •The second thermodynamics law.
- •Coulomb's law .
- •Electrostatic field.
- •Intensity of electrostatic field. Intensity of field created by point charge.
- •Electric field lines of force.
- •Principle or superposition.
- •Flux of a vector electric field intensity. Gauss theorem for electrostatic field.
- •Electric capacitance of a conductor.
- •Capacitor. Capacitance of plane capacitor.
- •Connection of capacitors in parallel and in series.
- •Energy of electric field.
- •Potential, potential difference.
- •Work in electrostatic field.
- •Joule law.
- •Power of electric current.
- •Ohm's law for non-uniform segments of electric circuit. Ohm's law for closed circuit.
Barometrical formula.
The barometric formula, sometimes called the exponential atmosphere or isothermal atmosphere, is a formula used to model how the pressure (or density) of the air changes withaltitude.
Boltsman's distribution.
The Boltzmann distribution for the fractional number of particles Ni / N occupying a set of states i possessing energy Ei is:
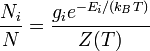
where ![]() is
the Boltzmann
constant, T is
temperature (assumed to be a well-defined quantity),
is
the Boltzmann
constant, T is
temperature (assumed to be a well-defined quantity), ![]() is
the degeneracy (meaning, the number of levels having energy
is
the degeneracy (meaning, the number of levels having energy ![]() ;
sometimes, the more general 'states' are used instead of levels, to
avoid using degeneracy in the equation), N is
the total number of particles and Z(T)
is the partition
function.
;
sometimes, the more general 'states' are used instead of levels, to
avoid using degeneracy in the equation), N is
the total number of particles and Z(T)
is the partition
function.
![]()
![]()
Diffusion.
Diffusion is one of several transport phenomena that occur in nature. A distinguishing feature of diffusion is that it results in mixing or mass transport without requiring bulk motion. Thus, diffusion should not be confused with convection or advection, which are other transport mechanisms that use bulk motion to move particles from one place to another.
Internal friction.
Internal friction is the force resisting motion between the elements making up a solid material while it undergoes plastic deformation.
Plastic deformation in solids is an irreversible change in the internal molecular structure of an object. This change may be due to either (or both) an applied force or a change in temperature. The change of an object's shape is called strain. The force causing it is called stress. Stress does not necessarily cause permanent change. As deformation occurs, internal forces oppose the applied force. If the applied stress is not too large these opposing forces may completely resist the applied force, allowing the object to assume a new equilibrium state and to return to its original shape when the force is removed. This is what is known in the literature as elastic deformation (or elasticity). Larger forces in excess of the elastic limit may cause a permanent (irreversible) deformation of the object. This is what is known as plastic deformation.
Heat conduction.
In heat transfer, conduction (or heat conduction) is the transfer of heat energy by microscopic diffusion and collisions of particles or quasi-particles within a body due to atemperature gradient. The microscopically diffusing and colliding objects include molecules, electrons, atoms, and phonons. They transfer microscopically disorganized kinetic and potential energy, which are jointly known as internal energy. Conduction takes place in all forms of ponderable matter, such as solids, liquids, gases and plasmas.
By conduction, as well as by thermal radiation, heat spontaneously flows from a body at a higher temperature to a body at a lower temperature. In the absence of external driving fluxes, temperature differences, over time, approach thermal equilibrium.
The first thermodynamics law. Its application for Isoprocess. Gas work at constant pressure and constant temperature.
The first explicit statement of the first law of thermodynamics, by Rudolf Clausius in 1850, referred to cyclic thermodynamic processes.
"In all cases in which work is produced by the agency of heat, a quantity of heat is consumed which is proportional to the work done; and conversely, by the expenditure of an equal quantity of work an equal quantity of heat is produced."[1]
Clausius stated the law also in another form, this time referring to the existence of a function of state of the system called the internal energy, and expressing himself in terms of a differential equation for the increments of a thermodynamic process. This equation may be translated into words as follows:
In a thermodynamic process of a closed system, the increment in the internal energy is equal to the difference between the increment of heat accumulated by the system and the increment of work done by it.[2]
The first law of thermodynamics may be stated thus:
Increase in internal energy of a body = heat supplied to the body - work done by the body. U = Q - W
For a thermodynamic cycle, the net heat supplied to the system equals the net work done by the system.
More specifically, the First Law encompasses several principles:
The law of conservation of energy.
This states that energy can be neither created nor destroyed. However, energy can change forms, and energy can flow from one place to another. The total energy of anisolated system remains the same.
The concept of internal energy and its relationship to temperature.
If a system, for example a rock, has a definite temperature, then its total energy has three distinguishable components. If the rock is flying through the air, it has kinetic energy. If it is high above the ground, it has gravitational potential energy. In addition to these, it has internal energy which is the sum of the kinetic energy of vibrations of the atoms in the rock, and other sorts of microscopic motion, and of the potential energy of interactions between the atoms within the rock. Other things being equal, the internal energy increases as the rock's temperature increases. The concept of internal energy is the characteristic distinguishing feature of the first law of thermodynamics.
The flow of heat is a form of energy transfer.
In other words, a quantity of heat that flows from a hot body to a cold one can be expressed as an amount of energy being transferred from the hot body to the cold one.
Performing work is a form of energy transfer.
For example, when a machine lifts a heavy object upwards, some energy is transferred from the machine to the object. The object acquires its energy in the form ofgravitational potential energy in this example.
Combining these principles leads to one traditional statement of the first law of thermodynamics: it is not possible to construct a perpetual motion machine which will continuously do work without consuming energy.
[edit]
