
- •Energy resources
- •Санкт-петербург
- •Предисловие
- •Energy resources Text 1
- •Sources of energy in the earth system
- •What Are Oil and Gas?
- •Where Do Oil and Gas Form?
- •Text 3 hydrocarbon systems: the making of a reserve
- •Source Rocks and Hydrocarbon Generation
- •Reservoir Rocks and Hydrocarbon Migration
- •Traps and Seals
- •Types of Oil and Gas Traps
- •Task 2. Read the text and discuss different types of oil and gas traps.
- •Text 5 birth of the oil industry in the usa
- •Text 6 oil exploration and production
- •The modern search for oil
- •Text 7 alternative reserves of hydrocarbons
- •Additional reading energy choices, energy problems the crisis of the 1970s
- •The oil crunch
- •Can other energy sources meet the need?
- •Environmental issues
- •Review questions
- •References
- •Contents
What Are Oil and Gas?
For reasons of economics and convenience, industrialized societies today rely primarily on oil (petroleum) and natural gas for their energy needs. Oil and natural gas consist of hydrocarbons, chainlike or ring-like molecules made of carbon and hydrogen atoms. For example, bottled gas (propane) has the chemical formula C3H9. Chemists consider hydrocarbons to be a type of organic chemical, so named because similar chemicals make up living organisms.
Some hydrocarbons are gaseous and invisible, some resemble watery liquids, some appear syrupy, and some are solid (Fig. 2). The viscosity (ability to flow) and the volatility (ability to evaporate) of a hydrocarbon product depend on the size of its molecules. Hydrocarbon products composed of short chains of molecules tend to be less viscous (they can flow more easily) and more volatile (they evaporate more easily) than products composed of long chains, simply because the long chains tend to tangle up with each other. Thus, short-chain molecules occur in gaseous form at room temperature, moderate-length-chain molecules occur in liquid form, and long-chain molecules occur in solid form as tar.
Why can we use hydrocarbons as fuel? Simply because hydrocarbons, like wood, burn—they react with oxygen to form carbon dioxide, water, and heat. As an example, we can describe the burning of gasoline by the reaction
2C8H18 + 25O2→16CO2 + 18H2O + heat energy
During such reactions, the potential energy stored in the chemical bonds of the hydrocarbon molecules converts into usable heat energy.
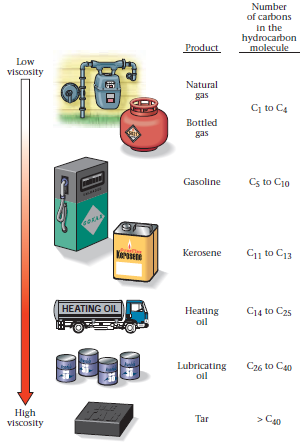
FIGURE 2. The diversity of hydrocarbon products we use: natural gas piped to houses for heating and cooking, bottled gas (propane), gasoline for cars, kerosene for heating or illumination, diesel fuel for trucks, lubricating oil for motors, and solid tar, which melts when heated and can be used to make asphalt. Note that these products are listed in order of increasing viscosity.
Where Do Oil and Gas Form?
Many people incorrectly believe that hydrocarbons come from buried trees or the carcasses of dinosaurs. In fact, the primary sources of the organic chemicals in oil and gas are dead algae and plankton. (Plankton, as we have seen, are the tiny plants and animals—typically around 0.5 mm in diameter—that float in sea or lake water.) When algae and plankton die, they settle to the bottom of a lake or sea. Because their cells are so tiny, they can be deposited only in quiet-water environments in which clay also settles, so typically the cells mix with clay to create organic-rich, muddy ooze. For this ooze to be preserved, it must be deposited in oxygen-poor water. Otherwise, the organic chemicals in the ooze would react with oxygen or be eaten by bacteria, and thus would decompose quickly and disappear. In some quiet-water environments (oceans, lagoons, or lakes), dead algae and plankton can get buried by still more sediment before being destroyed. Eventually, the resulting ooze lithifies and becomes black organic shale (in contrast to ordinary shale that consists only of clay), which contains the raw materials from which hydrocarbons eventually form. Thus, we refer to organic shale as a source rock.
If organic shale is buried deeply enough (2 to 4 km), it becomes warmer, since temperature increases with depth in the Earth. Chemical reactions slowly transform the organic material in the shale into waxy molecules called kerogen (Fig. 3).
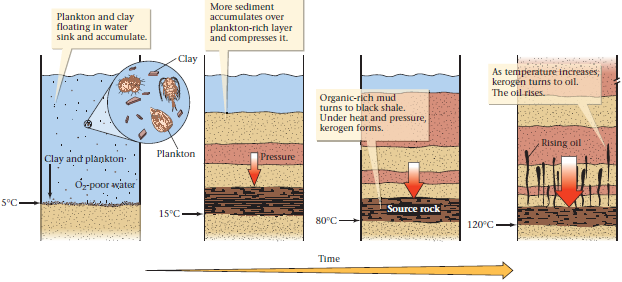
FIGURE 3. Plankton, algae, and clay settle out of water and become progressively buried and compacted, gradually being transformed into black organic shale. When heated for a long time, the organic matter in black shale is transformed into oil shale, which contains kerogen. Eventually, the kerogen transforms into oil and gas. The oil may then start to seep upward out of the shale. The red arrow indicates pressure, which increases as more sediment accumulates above.
Shale containing kerogen is called oil shale. If the oil shale warms to temperatures of greater than about 90°C, the kerogen molecules break down to form oil and natural gas molecules. At temperatures over about 160°C, any remaining oil breaks down to form natural gas, and at temperatures over 250°C, organic matter transforms into graphite. Thus, oil itself forms only in a relatively narrow range of temperatures, called the oil window (Fig. 4).
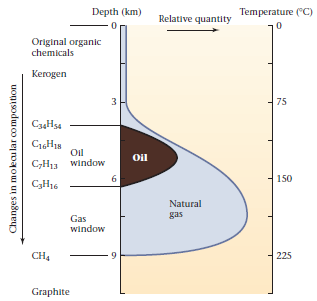
FIGURE 4.14.5 The oil window is the range of temperature conditions (i.e., depth) at which hydrocarbons form. In regions with a geothermal gradient of 25°C per km, oil occurs only at depths of less than about 6.5 km, the gradient portrayed on this graph, Note that gas can be found at greater depths. The length of hydrocarbon chains decreases with increasing depth, because at higher temperatures longer chains break to form smaller ones.
For regions with a geothermal gradient of 25°C/km, the oil window lies at depths of about 3.5 to 6.5 km, whereas gas can survive down to 9 km. If the geothermal gradient is low (15°C/km), oil survives only below about 11 km. Thus, hydrocarbon reserves can only exist in the topmost 15 to 25% of the crust.
Task 2. Match the following words and word combination and their definitions:
A. Gasoline
B. Kerosene
C. Diesel fuel
D. Lubricating oil
E. Asphalt
F. Ooze
G. Source rock
H. Kerogen
I. Oil shale
J. Oil window
1. Black or brown petroleum-like material that has a consistency varying from viscous liquid to glassy solid. It is obtained either as a residue from the distillation of petroleum or from natural deposits. It consists of compounds of hydrogen and carbon with minor proportions of nitrogen, sulfur, and oxygen. It softens when heated and is elastic under certain conditions. Used principally in road surfacing, asphalt is also used for roofs, coatings, floor tilings, and waterproofing, and in industrial products.
2. Rock from which fragments have been derived which form a later, usually sedimentary rock. Also known as mother rock; parent rock.
3. Fuel used for internal combustion in diesel engines; usually that fraction of crude oil that distills after kerosene.
4. An organic-rich fine-grained sedimentary rock, contains significant amounts of kerogen (a solid mixture of organic chemical compounds) from which liquid hydrocarbons can be extracted.
5. A light, volatile mixture of hydrocarbons for use in the internal-combustion engine and as an organic solvent, obtained primarily by fractional distillation and "cracking" of petroleum, but also obtained from natural gas, by destructive distillation of oil shales and coal, and by a process that converts methanol to gasoline using zeolite as a catalyst.
6. A fine-grained calcareous or siliceous marine deposit consisting of the hard parts of planktonic organisms.
7. Selected fractions of refined petroleum or other oils (with or without additives) used to lessen friction between moving surfaces. Also known as lube oil.
8. A mixture of organic chemical compounds that make up a portion of the organic matter in sedimentary rocks. It is insoluble in normal organic solvents because of the huge molecular weight of its component compounds. The soluble portion is known as bitumen.
9. Organic compound, a clear, oily, highly flammable liquid with a strong odour, distilled from petroleum (10–25% of total volume). It is a mixture of about 10 different types of fairly simple hydrocarbons, depending on its source. It is less volatile than gasoline, boiling at 140–320 °C. It is burned in lamps, heaters, and furnaces and is used as a fuel or fuel component for diesel and tractor engines, jet engines, and rockets and as a solvent for greases and insecticides.
10. A temperature dependant interval in the subsurface where oil is generated and expelled from the source rocks.
Task 3. Look at Fig.2 and discuss in groups the main characteristics of hydrocarbon products we use.
Task 4. Discuss what you have learnt about the process of oil and gas formation with the help of Fig.3.
Task 5. Read the text and answer the following questions:
1. What are oil and gas?
2. Where do oil and gas form?
3. What is oil shale?
