
- •ICU Protocols
- •Preface
- •Acknowledgments
- •Contents
- •Contributors
- •1: Airway Management
- •Suggested Reading
- •2: Acute Respiratory Failure
- •Suggested Reading
- •Suggested Reading
- •Website
- •4: Basic Mechanical Ventilation
- •Suggested Reading
- •Suggested Reading
- •Websites
- •Suggested Reading
- •Websites
- •7: Weaning
- •Suggested Reading
- •8: Massive Hemoptysis
- •Suggested Reading
- •9: Pulmonary Thromboembolism
- •Suggested Reading
- •Suggested Reading
- •Websites
- •11: Ventilator-Associated Pneumonia
- •Suggested Readings
- •12: Pleural Diseases
- •Suggested Reading
- •Websites
- •13: Sleep-Disordered Breathing
- •Suggested Reading
- •Websites
- •14: Oxygen Therapy
- •Suggested Reading
- •15: Pulse Oximetry and Capnography
- •Conclusion
- •Suggested Reading
- •Websites
- •16: Hemodynamic Monitoring
- •Suggested Reading
- •Websites
- •17: Echocardiography
- •Suggested Readings
- •Websites
- •Suggested Reading
- •Websites
- •19: Cardiorespiratory Arrest
- •Suggested Reading
- •Websites
- •20: Cardiogenic Shock
- •Suggested Reading
- •21: Acute Heart Failure
- •Suggested Reading
- •22: Cardiac Arrhythmias
- •Suggested Reading
- •Website
- •23: Acute Coronary Syndromes
- •Suggested Reading
- •Website
- •Suggested Reading
- •25: Aortic Dissection
- •Suggested Reading
- •26: Cerebrovascular Accident
- •Suggested Reading
- •Websites
- •27: Subarachnoid Hemorrhage
- •Suggested Reading
- •Websites
- •28: Status Epilepticus
- •Suggested Reading
- •29: Acute Flaccid Paralysis
- •Suggested Readings
- •30: Coma
- •Suggested Reading
- •Suggested Reading
- •Websites
- •32: Acute Febrile Encephalopathy
- •Suggested Reading
- •33: Sedation and Analgesia
- •Suggested Reading
- •Websites
- •34: Brain Death
- •Suggested Reading
- •Websites
- •35: Upper Gastrointestinal Bleeding
- •Suggested Reading
- •36: Lower Gastrointestinal Bleeding
- •Suggested Reading
- •37: Acute Diarrhea
- •Suggested Reading
- •38: Acute Abdominal Distension
- •Suggested Reading
- •39: Intra-abdominal Hypertension
- •Suggested Reading
- •Website
- •40: Acute Pancreatitis
- •Suggested Reading
- •Website
- •41: Acute Liver Failure
- •Suggested Reading
- •Suggested Reading
- •Websites
- •43: Nutrition Support
- •Suggested Reading
- •44: Acute Renal Failure
- •Suggested Reading
- •Websites
- •45: Renal Replacement Therapy
- •Suggested Reading
- •Website
- •46: Managing a Patient on Dialysis
- •Suggested Reading
- •Websites
- •47: Drug Dosing
- •Suggested Reading
- •Websites
- •48: General Measures of Infection Control
- •Suggested Reading
- •Websites
- •49: Antibiotic Stewardship
- •Suggested Reading
- •Website
- •50: Septic Shock
- •Suggested Reading
- •51: Severe Tropical Infections
- •Suggested Reading
- •Websites
- •52: New-Onset Fever
- •Suggested Reading
- •Websites
- •53: Fungal Infections
- •Suggested Reading
- •Suggested Reading
- •Website
- •55: Hyponatremia
- •Suggested Reading
- •56: Hypernatremia
- •Suggested Reading
- •57: Hypokalemia and Hyperkalemia
- •57.1 Hyperkalemia
- •Suggested Reading
- •Website
- •58: Arterial Blood Gases
- •Suggested Reading
- •Websites
- •59: Diabetic Emergencies
- •59.1 Hyperglycemic Emergencies
- •59.2 Hypoglycemia
- •Suggested Reading
- •60: Glycemic Control in the ICU
- •Suggested Reading
- •61: Transfusion Practices and Complications
- •Suggested Reading
- •Websites
- •Suggested Reading
- •Website
- •63: Onco-emergencies
- •63.1 Hypercalcemia
- •63.2 ECG Changes in Hypercalcemia
- •63.3 Superior Vena Cava Syndrome
- •63.4 Malignant Spinal Cord Compression
- •Suggested Reading
- •64: General Management of Trauma
- •Suggested Reading
- •65: Severe Head and Spinal Cord Injury
- •Suggested Reading
- •Websites
- •66: Torso Trauma
- •Suggested Reading
- •Websites
- •67: Burn Management
- •Suggested Reading
- •68: General Poisoning Management
- •Suggested Reading
- •69: Syndromic Approach to Poisoning
- •Suggested Reading
- •Websites
- •70: Drug Abuse
- •Suggested Reading
- •71: Snakebite
- •Suggested Reading
- •72: Heat Stroke and Hypothermia
- •72.1 Heat Stroke
- •72.2 Hypothermia
- •Suggested Reading
- •73: Jaundice in Pregnancy
- •Suggested Reading
- •Suggested Reading
- •75: Severe Preeclampsia
- •Suggested Reading
- •76: General Issues in Perioperative Care
- •Suggested Reading
- •Web Site
- •77.1 Cardiac Surgery
- •77.2 Thoracic Surgery
- •77.3 Neurosurgery
- •Suggested Reading
- •78: Initial Assessment and Resuscitation
- •Suggested Reading
- •79: Comprehensive ICU Care
- •Suggested Reading
- •Website
- •80: Quality Control
- •Suggested Reading
- •Websites
- •81: Ethical Principles in End-of-Life Care
- •Suggested Reading
- •82: ICU Organization and Training
- •Suggested Reading
- •Website
- •83: Transportation of Critically Ill Patients
- •83.1 Intrahospital Transport
- •83.2 Interhospital Transport
- •Suggested Reading
- •84: Scoring Systems
- •Suggested Reading
- •Websites
- •85: Mechanical Ventilation
- •Suggested Reading
- •86: Acute Severe Asthma
- •Suggested Reading
- •87: Status Epilepticus
- •Suggested Reading
- •88: Severe Sepsis and Septic Shock
- •Suggested Reading
- •89: Acute Intracranial Hypertension
- •Suggested Reading
- •90: Multiorgan Failure
- •90.1 Concurrent Management of Hepatic Dysfunction
- •Suggested Readings
- •91: Central Line Placement
- •Suggested Reading
- •92: Arterial Catheterization
- •Suggested Reading
- •93: Pulmonary Artery Catheterization
- •Suggested Reading
- •Website
- •Suggested Reading
- •95: Temporary Pacemaker Insertion
- •Suggested Reading
- •96: Percutaneous Tracheostomy
- •Suggested Reading
- •97: Thoracentesis
- •Suggested Reading
- •98: Chest Tube Placement
- •Suggested Reading
- •99: Pericardiocentesis
- •Suggested Reading
- •100: Lumbar Puncture
- •Suggested Reading
- •Website
- •101: Intra-aortic Balloon Pump
- •Suggested Reading
- •Appendices
- •Appendix A
- •Appendix B
- •Common ICU Formulae
- •Appendix C
- •Appendix D: Syllabus for ICU Training
- •Index

Antibiotic Stewardship |
49 |
|
|
Subhash Todi and Rajesh Chawla |
|
A 70-year-old male patient had been admitted to the ICU with left-sided hemiparesis for 4 days. He was catheterized on admission and intubated for airway protection. He had spiked a fever of 102°F with chills and became drowsy. A broad-spectrum antibiotic was started empirically after sending blood cultures.
Judicious antibiotic prescription along with infection control is the cornerstone for preventing emergence of drug-resistant organisms in the ICU. Appropriate (right choice) and adequate (right time, right dose) antibiotic coverage improves outcome in infected patients.
Step 1: Formulate a plan for antibiotic selection
•Antibiotics in the ICU are given either empirically for presumed infection with culture report pending, or prophylactically mainly perioperative or definitively when infection is documented with positive culture results.
•Reason for antibiotic selection should be clearly documented in the antibiotic order form, which should be audited periodically for correctness.
•Appropriate antibiotics should be chosen depending on local epidemiology and resistance pattern.
S. Todi, M.D., M.R.C.P. (*)
Critical Care & Emergency, A.M.R.I. Hospital, Kolkata, West Bengal, India e-mail: drsubhashtodi@gmail.com
R. Chawla, M.D., F.C.C.M.
Department of Respiratory, Critical Care & Sleep Medicine, Indraprastha Apollo Hospitals, New Delhi, New Delhi, India
e-mail: drchawla@hotmail.com
R. Chawla and S. Todi (eds.), ICU Protocols: A stepwise approach, |
389 |
DOI 10.1007/978-81-322-0535-7_49, © Springer India 2012 |
|

390 |
S. Todi and R. Chawla |
|
|
Step 2: Send appropriate cultures
•This should ideally be done prior to starting antibiotics.
•Blood, urine, sputum, and endotracheal secretion should be promptly transported to the microbiology laboratory and expeditiously processed.
Step 3: Start antibiotics early
•Every hour delay in starting effective antibiotics from the onset of septic shock increases the risk of death from sepsis by 6–10%.
•Antibiotics should be started within 1 h of recognition of septic shock as “Time is tissue.”
Step 4: Choose empirical antibiotics appropriately
•Empirical antibiotics should be chosen carefully as initial wrong choice increases mortality, even if the antibiotic is changed appropriately after culture results are obtained.
•Initial antibiotic choice should be based on the patient’s history, underlying disease or clinical syndrome, susceptibility pattern of the pathogen in the community and hospital, and previous colonization pattern.
•Recently used antibiotic class should generally be avoided.
•Choose broad-spectrum antibiotics that have activity against most likely bacterial pathogens.
Step 5: Stratify the risk of infection with drug-resistant organisms (Table 49.1)
•The patient should be assessed for risk of infection with multidrug-resistant bacteria.
•If one or more risk factors are present, antibiotic choice should be broadened to cover these organisms.
Table 49.1 Risk factors for drug-resistant bacteria
Antimicrobial therapy in preceding 90 days
Current hospitalization of 5 days or more
High frequency of antibiotic resistance in the community or in the specific hospital unit
Immunosuppressive disease and/or therapy
Hospitalization for 2 days or more in preceding 90 days
Residence in the nursing home or extended care facility
Chronic dialysis within 30 days
Home infusion therapy (including antibiotic)
Home wound care
Having a family member with a recent history of infection with multidrug-resistant pathogens
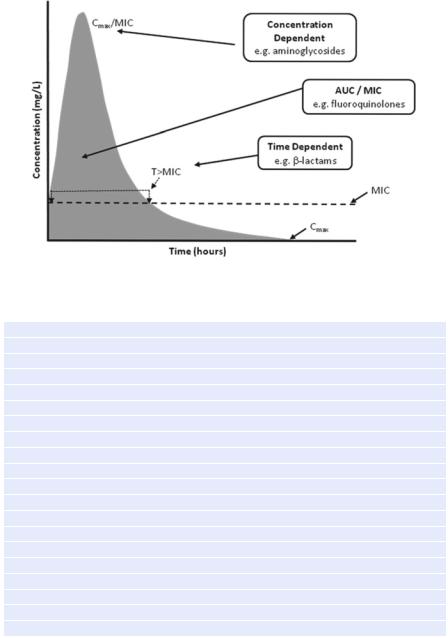
Step 6: Follow pharmacokinetic and pharmacodynamic principles while prescribing antibiotics (Fig. 49.1)
•Give adequate intravenous dose (Table 49.2).
•Give antibiotics that penetrate in adequate concentrations into the presumed source of sepsis.
49 Antibiotic Stewardship |
391 |
|
|
Fig. 49.1 Pharmacokinetic and pharmacodynamic principles
Table 49.2 Appropriate doses of common antibiotics (Refer to Appendix 1)
Antipseudomonal cephalosporin
Cefepime, 1–2 g every 8–12 h
Ceftazidime, 2 g every 8 h
Carbapenems
Imipenem, 500 mg every 6 h or 1 g every 8 h
Meropenem, 1 g every 8 h
Doripenem, 500 mg every 8 h
Ertapenem, 1 g once daily
b-Lactam/b-lactamase inhibitor
Piperacillin–tazobactam, 4.5 g every 6 h
Aminoglycosides
Gentamicin, 7 mg/kg/day
Tobramycin, 7 mg/kg/day
Amikacin, 20 mg/kg/day
Antipseudomonal quinolones
Levofloxacin, 750 mg/day
Ciprofloxacin, 400 mg every 8 h
Vancomycin, 15 mg/kg every 12 h
Linezolid, 600 mg every 12 h
Colistin: 1–2 million units every 8 h
Dosages are based on normal renal and hepatic function
Peak drug levels for gentamicin and tobramycin should be 8–10 mcg/mL and trough levels less than 2 mcg/mL
Peak drug levels for amikacin should be 25–30 mcg/mL and trough levels should be less than 4–8 mcg/mL
Trough levels for vancomycin should be 15–20 mcg/mL
392 |
S. Todi and R. Chawla |
|
|
•Time-dependent antibiotics like b-lactams (maximum bacterial inhibition depends on time above minimum inhibitory concentration) should be given as a continuous infusion.
•Dose-dependent antibiotics like aminoglycoside (maximum bacterial inhibition depends on peak antibiotic concentration) should be given as a once-daily bolus dose.
•Adjust the dose of antibiotics depending on renal and hepatic dysfunction.
Step 7: Assess the patient daily and de-escalate antibiotics once culture results are obtained
•Clinical response should be assessed frequently, and if the patient is responding favorably, antibiotics should be de-escalated to a narrower spectrum, and unnecessary antibiotics should be stopped if culture results permit.
•Decisions to continue, narrow, or stop antimicrobial therapy must be made on the basis of clinician judgment and laboratory information like decrease in leukocytosis, decreasing C reactive protein, and a low procalcitonin level.
Step 8: Consider the combination of antibiotics in specific situations
•The combination of antibiotics (two appropriate antibiotics against the same organism) is indicated in difficult to treat multidrug-resistant pathogens like Acinetobacter and Pseudomonas sp. Combination therapy is also indicated for neutropenic patient with severe sepsis and selected patients with severe Pseudomonas infection with respiratory failure and shock. Similarly, a combination of beta-lactam and macrolide is recommended for pneumococcal bacteremia.
Step 9: Decide on duration of antibiotic therapy
•Duration of therapy should typically be 7–10 days.
•Longer courses may be appropriate in patients who have a slow clinical response, undrainable foci of infection, or immunologic deficiencies including neutropenia.
•If culture result is negative and there is a favorable clinical response, most antibiotics can be stopped in 5 days.
•In Pseudomonas and Acinetobacter infection, severe sepsis should be treated for 2 weeks.
Step 10: Implement antibiotic stewardship program
•Constitute an antibiotic stewardship team along with the microbiologist, infection control nurse, infectious disease consultant, and clinical pharmacist.
•Educating ICU staff the principles of antibiotic stewardship is of prime importance.
•Proper utilization of local antibiogram should be done.
•Utilize optimally the information obtained from the microbiology laboratory.
•Work in close collaboration with microbiologists and other physicians involved in antibiotic prescribing.
