
- •ICU Protocols
- •Preface
- •Acknowledgments
- •Contents
- •Contributors
- •1: Airway Management
- •Suggested Reading
- •2: Acute Respiratory Failure
- •Suggested Reading
- •Suggested Reading
- •Website
- •4: Basic Mechanical Ventilation
- •Suggested Reading
- •Suggested Reading
- •Websites
- •Suggested Reading
- •Websites
- •7: Weaning
- •Suggested Reading
- •8: Massive Hemoptysis
- •Suggested Reading
- •9: Pulmonary Thromboembolism
- •Suggested Reading
- •Suggested Reading
- •Websites
- •11: Ventilator-Associated Pneumonia
- •Suggested Readings
- •12: Pleural Diseases
- •Suggested Reading
- •Websites
- •13: Sleep-Disordered Breathing
- •Suggested Reading
- •Websites
- •14: Oxygen Therapy
- •Suggested Reading
- •15: Pulse Oximetry and Capnography
- •Conclusion
- •Suggested Reading
- •Websites
- •16: Hemodynamic Monitoring
- •Suggested Reading
- •Websites
- •17: Echocardiography
- •Suggested Readings
- •Websites
- •Suggested Reading
- •Websites
- •19: Cardiorespiratory Arrest
- •Suggested Reading
- •Websites
- •20: Cardiogenic Shock
- •Suggested Reading
- •21: Acute Heart Failure
- •Suggested Reading
- •22: Cardiac Arrhythmias
- •Suggested Reading
- •Website
- •23: Acute Coronary Syndromes
- •Suggested Reading
- •Website
- •Suggested Reading
- •25: Aortic Dissection
- •Suggested Reading
- •26: Cerebrovascular Accident
- •Suggested Reading
- •Websites
- •27: Subarachnoid Hemorrhage
- •Suggested Reading
- •Websites
- •28: Status Epilepticus
- •Suggested Reading
- •29: Acute Flaccid Paralysis
- •Suggested Readings
- •30: Coma
- •Suggested Reading
- •Suggested Reading
- •Websites
- •32: Acute Febrile Encephalopathy
- •Suggested Reading
- •33: Sedation and Analgesia
- •Suggested Reading
- •Websites
- •34: Brain Death
- •Suggested Reading
- •Websites
- •35: Upper Gastrointestinal Bleeding
- •Suggested Reading
- •36: Lower Gastrointestinal Bleeding
- •Suggested Reading
- •37: Acute Diarrhea
- •Suggested Reading
- •38: Acute Abdominal Distension
- •Suggested Reading
- •39: Intra-abdominal Hypertension
- •Suggested Reading
- •Website
- •40: Acute Pancreatitis
- •Suggested Reading
- •Website
- •41: Acute Liver Failure
- •Suggested Reading
- •Suggested Reading
- •Websites
- •43: Nutrition Support
- •Suggested Reading
- •44: Acute Renal Failure
- •Suggested Reading
- •Websites
- •45: Renal Replacement Therapy
- •Suggested Reading
- •Website
- •46: Managing a Patient on Dialysis
- •Suggested Reading
- •Websites
- •47: Drug Dosing
- •Suggested Reading
- •Websites
- •48: General Measures of Infection Control
- •Suggested Reading
- •Websites
- •49: Antibiotic Stewardship
- •Suggested Reading
- •Website
- •50: Septic Shock
- •Suggested Reading
- •51: Severe Tropical Infections
- •Suggested Reading
- •Websites
- •52: New-Onset Fever
- •Suggested Reading
- •Websites
- •53: Fungal Infections
- •Suggested Reading
- •Suggested Reading
- •Website
- •55: Hyponatremia
- •Suggested Reading
- •56: Hypernatremia
- •Suggested Reading
- •57: Hypokalemia and Hyperkalemia
- •57.1 Hyperkalemia
- •Suggested Reading
- •Website
- •58: Arterial Blood Gases
- •Suggested Reading
- •Websites
- •59: Diabetic Emergencies
- •59.1 Hyperglycemic Emergencies
- •59.2 Hypoglycemia
- •Suggested Reading
- •60: Glycemic Control in the ICU
- •Suggested Reading
- •61: Transfusion Practices and Complications
- •Suggested Reading
- •Websites
- •Suggested Reading
- •Website
- •63: Onco-emergencies
- •63.1 Hypercalcemia
- •63.2 ECG Changes in Hypercalcemia
- •63.3 Superior Vena Cava Syndrome
- •63.4 Malignant Spinal Cord Compression
- •Suggested Reading
- •64: General Management of Trauma
- •Suggested Reading
- •65: Severe Head and Spinal Cord Injury
- •Suggested Reading
- •Websites
- •66: Torso Trauma
- •Suggested Reading
- •Websites
- •67: Burn Management
- •Suggested Reading
- •68: General Poisoning Management
- •Suggested Reading
- •69: Syndromic Approach to Poisoning
- •Suggested Reading
- •Websites
- •70: Drug Abuse
- •Suggested Reading
- •71: Snakebite
- •Suggested Reading
- •72: Heat Stroke and Hypothermia
- •72.1 Heat Stroke
- •72.2 Hypothermia
- •Suggested Reading
- •73: Jaundice in Pregnancy
- •Suggested Reading
- •Suggested Reading
- •75: Severe Preeclampsia
- •Suggested Reading
- •76: General Issues in Perioperative Care
- •Suggested Reading
- •Web Site
- •77.1 Cardiac Surgery
- •77.2 Thoracic Surgery
- •77.3 Neurosurgery
- •Suggested Reading
- •78: Initial Assessment and Resuscitation
- •Suggested Reading
- •79: Comprehensive ICU Care
- •Suggested Reading
- •Website
- •80: Quality Control
- •Suggested Reading
- •Websites
- •81: Ethical Principles in End-of-Life Care
- •Suggested Reading
- •82: ICU Organization and Training
- •Suggested Reading
- •Website
- •83: Transportation of Critically Ill Patients
- •83.1 Intrahospital Transport
- •83.2 Interhospital Transport
- •Suggested Reading
- •84: Scoring Systems
- •Suggested Reading
- •Websites
- •85: Mechanical Ventilation
- •Suggested Reading
- •86: Acute Severe Asthma
- •Suggested Reading
- •87: Status Epilepticus
- •Suggested Reading
- •88: Severe Sepsis and Septic Shock
- •Suggested Reading
- •89: Acute Intracranial Hypertension
- •Suggested Reading
- •90: Multiorgan Failure
- •90.1 Concurrent Management of Hepatic Dysfunction
- •Suggested Readings
- •91: Central Line Placement
- •Suggested Reading
- •92: Arterial Catheterization
- •Suggested Reading
- •93: Pulmonary Artery Catheterization
- •Suggested Reading
- •Website
- •Suggested Reading
- •95: Temporary Pacemaker Insertion
- •Suggested Reading
- •96: Percutaneous Tracheostomy
- •Suggested Reading
- •97: Thoracentesis
- •Suggested Reading
- •98: Chest Tube Placement
- •Suggested Reading
- •99: Pericardiocentesis
- •Suggested Reading
- •100: Lumbar Puncture
- •Suggested Reading
- •Website
- •101: Intra-aortic Balloon Pump
- •Suggested Reading
- •Appendices
- •Appendix A
- •Appendix B
- •Common ICU Formulae
- •Appendix C
- •Appendix D: Syllabus for ICU Training
- •Index
Sedation and Analgesia |
33 |
|
Ashwin Kumar Mani, Nagarajan Ramakrishnan,
and Sheila Nainan Myatra
A 48-year-old male patient was admitted to the ICU with acute respiratory distress syndrome secondary to H1N1 inßuenza. He was noted to be markedly hypoxemic requiring high FiO2. He was intubated and put on a ventilator. He was tachypneic, tachycardic, and restless and was breathing at the rate of 40 per min and struggling with the ventilator.
Critically ill patients on mechanical ventilation are frequently managed with sedative and analgesics. Pain is a very common experience in the ICU. Failure to recognize pain leads to agitation and increase use of sedatives. Majority of patients Þnd even routine ICU procedures uncomfortable and painful. Inability to communicate about pain due to presence of an endotracheal tube may lead to high levels of anxiety and may precipitate development of posttraumatic stress disorder. Now it is recommended to aggressively manage pain.
Step 1: Identify the need for sedation and analgesia (Table 33.1)
¥Appropriate analgesia might obviate the need for sedation or decrease the sedation needs.
¥In certain groups of patients, sedation is very important to prevent them from selfharm (removing lines) or to decrease the work of breathing and oxygen demand.
¥Common indications of analgesia and sedation (Table 33.1).
A.K. Mani, A.B.(I.M.), A.B.( Pulm. & C.C.M.) (*)
Critical Care Medicine, Apollo First Med Hospital, Chennai, India e-mail: ashwin@icuconsultants.com
N. Ramakrishnan, A.B.(I.M.), F.A.C.P.
Department of Critical Care Services, Apollo Hospitals, Chennai, India
S.N. Myatra, M.D.
Department of Anaesthesia, Critical Care and Pain, Tata Memorial Hospital, Mumbai, India
R. Chawla and S. Todi (eds.), ICU Protocols: A stepwise approach, |
265 |
DOI 10.1007/978-81-322-0535-7_33, © Springer India 2012 |
|

266 |
A.K. Mani et al. |
|
|
Table 33.1 Indications of sedation and analgesia
Facilitation of endotracheal intubation and mechanical ventilation (for amnesia, anxiolysis, reduction of agitation, toleration of endotracheal tube (ETT) and to reduce cough, to alleviate dyspnea, to minimize pain, and to prevent self-extubation)
While performing invasive procedures
To facilitate nursing care
Postoperative/trauma pain
To tolerate ICU environmental inßuences (stressful environment, monitoring devices, noise, fear of illness, separation from family)
During severe hypoxemia (low tidal volume ventilation, high-frequency oscillation (HFO), and prone positioning)
To bear underlying or hospital-acquired medical illnesses
Step 2: Consider the use of commonly used sedatives and analgesics (Table 33.2)
Step 3: Choose an appropriate agent
¥There is no ideal agent for ICU sedation.
¥An ideal sedative and analgesic regimen should provide adequate sedation and pain control, rapid onset of action, and allow rapid recovery after discontinuation, minimal adverse effects, minimal systemic accumulation, and minimal delirium without increasing overall health-care costs.
¥In different clinical scenarios, some agents have been shown to be better than others. Consider the following before choosing an agent:
ÐAll patients need analgesia with or without sedation. Recent studies have shown that minimal use of continuous sedation is feasible without apparent adverse effects.
ÐHave a patient-focused approach. Select analgesic and sedative drugs based on patientsÕ needs.
¥Remember that the degree of sedation/anxiolysis and analgesia required varies between patients and in the same patient over time (physiotherapy, weaning).
¥Identify predisposing and precipitating factorsÑunderlying medical conditions such as chronic pain or arthritis, history of alcohol, or substance abuse. Psychiatric illness can inßuence medication. For example, patients who appear delirious or severely agitated in the ICU may become overmedicated with sedative drugs because their medications have been omitted.
¥Adequate analgesia should be ensured prior to sedating the patient.
¥In a nonintubated patient agitated due to pain, when opioid analgesics such as morphine or fentanyl are used, remember that they can cause respiratory depression. In patients with obstructive sleep apnea, even small doses should be used with caution.
¥For postoperative pain/trauma, epidural analgesia, if available, is a suitable option.
¥Gamma-aminobutyric acid agonists (benzodiazepines, propofol) are prone to cause acute cognitive dysfunction.

Table 33.2 Drug details for sedation and analgesia |
|
|
|
|||
|
Time |
Duration |
Presence |
|
|
|
|
to onset |
of effect of |
of active |
Accumulation |
|
|
Drug |
(min) |
one dose (h) |
metabolites |
in renal failure |
Dose (IV) |
Comments |
Fentanyl |
1Ð2 |
2Ð4 |
No |
No |
B+ = 50Ð100 mcg |
Reduces tachypnea |
|
|
|
|
|
|
Respiratory depression |
|
|
|
|
|
I+ = 0.7Ð10 mcg/kg/hr |
Accumulation in hepatic/renal failure |
Morphine |
5Ð10 |
2Ð4 |
Yes |
Yes |
B = 1Ð10 mg |
Same as other opioids |
|
|
|
|
|
I = 2Ð10 mg/h |
|
Remifentanil |
1Ð2 |
10Ð20 mt |
No |
No |
B = 1 mcg/kg |
Decreased heart rate and blood pressure |
|
|
|
|
|
I = 0.25Ð1.0 mcg/kg/min |
Increased intracranial pressure |
Hydromorphone |
5Ð10 |
2Ð4 |
Yes |
Yes |
B = 0.2Ð0.6 mg |
May work in patients tolerant to morphine and |
|
|
|
|
|
|
fentanyl, respiratory depression |
|
|
|
|
|
I = 0.5Ð3 mg/h |
Highly addictive |
Lorazepam |
5Ð20 |
6Ð8 |
No |
Yes |
B = 2Ð4 mg |
Propylene glycol toxicity (anion gap |
|
|
|
|
|
|
metabolic acidosis, renal insufÞciency) |
|
|
|
|
|
2Ð6 mg q4 to q6 |
Independent risk factor for delirium |
|
|
|
|
|
(duration too long for |
|
|
|
|
|
|
effective infusions) |
|
Midazolam |
2Ð10 |
1Ð4 |
Yes |
Yes |
B = 2Ð5 mg |
Many drug interactions |
|
|
|
|
|
I = 1Ð20 mg/h |
May increase midazolam levels |
Propofol |
30Ð50 s |
3Ð10 (dose |
No |
InsigniÞcant |
B = 0.2Ð2 |
Hypotension |
|
|
dependent) |
|
|
mg/kg (maximum 20 mg) |
Increased serum triglyceride |
|
|
|
|
|
I = 10Ð150 mcg/kg/min |
Propofol infusion syndrome (>5 mg/kg/h for |
|
|
|
|
|
|
more than 48 h) |
Dexmedetomidine |
30 |
4 |
No |
No |
B = 1 mg/kg (should be |
Sedative, anxiolysis, with analgesic property |
|
|
|
|
|
given over 15Ð20 min) |
(reduces opioid requirement by >50%) |
|
|
|
|
|
I = 0.2Ð0.7 mg/kg/h |
Notable adverse event is bradycardia |
B+ bolus dose, I+ infusion dose |
|
|
|
|
|
|
Analgesia and Sedation 33
267
268 |
A.K. Mani et al. |
|
|
¥Lorazepam is an independent risk factor for transitioning to delirium.
¥Sedation with dexmedetomidine causes less delirium than with midazolam or propofol.
¥Analgosedation or co-sedationÑoften analgesic medications are added to a sedative infusion (e.g., midazolam and fentanyl) with reduced doses of both agents and in some cases more effective therapy.
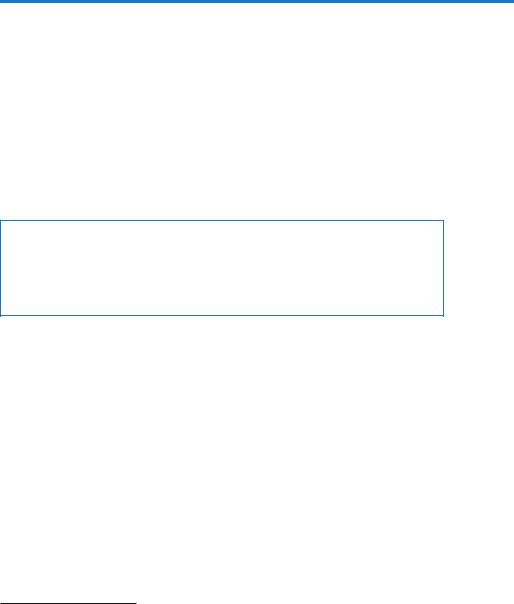
Pain (Refer Table 33.3)
Patient comfortable/pain free
Yes |
• |
Reassess goal daily |
|
• |
Titrate and taper therapy to |
|
|
maintaingoal |
|
• |
Perform awakening trial |
|
|
|
No
Nonintubated Intubated
Provide analgesia for postoperative/trauma/acute illness pain using one of the options below:
•Intermittent analgesic boluses of IV fentanyl/ morphine/NSAIDs, etc.
•Use PO analgesic if feasible
•IV PCA
•Epidural (if present)— analgesia with infusion/PCEA of local anesthetic and opioid combination
•Rule out/correct reversible causes
•Use nonpharmacologic treatment
•Optimize the environment
Hemodynamically stable (Step 3)
•Fentanyl
•Hydromorphone
•Morphine
Hemodynamically unstable
•Fentanyl
Renal impairment
•Fentanyl
•Hydromorphone
•Fentanyl 50 – 100 mcg bolus f/b 50 mcg/h. Revaluate in 15 minutes.
•If inadequate pain relief rebolus 50 – 100 mcg and increase drip by 50%.
•Revaluate again in 30 minutes and increase or decrease dose by 50% if inadequate or over sedation
Pain scores
Target CPOT=0–1 Numeric PS=3 (mild pain)
33 Sedation and Analgesia |
269 |
|
|
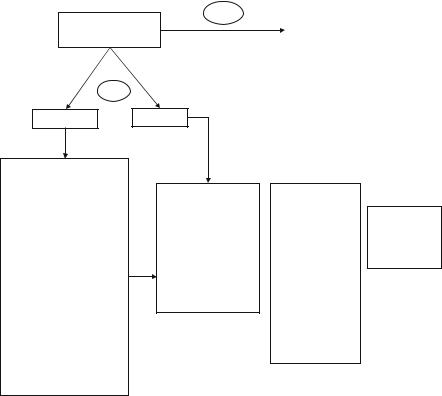
Sedation (Refer Table 33.4, 33.5, 33.6)
|
|
|
|
|
|
|
Anticipate sedation |
|
|
|
|
(<72 hours) |
|
|
|
|
• |
Midazolam |
|
Is patient |
|
• |
Propofol |
|
|
• |
Dexmedetomidine |
|
|
agitated / |
|
|
||
|
|
|
|
|
|
|
|
|
|
anxious? |
|
|
|
|
|
|
|
|
|
|
|
Anticipate sedation |
||
|
|
(>72 hours) |
||
|
|
• |
Midazolam |
|
|
|
• |
Lorazepam |
|
|
|
(Concerns of delirium |
||
|
|
|||
|
|
with long-term |
||
|
|
infusions) |
||
|
|
The patient has renal |
||
|
|
impairment |
||
|
|
• |
Lorazepam |
|
|
|
• |
Propofol |
|
|
|
• |
Consider |
|
|
|
|
intermittent boluses |
|
• Propofol 10–25 mcg/kg/min titrated in increments of 10– 25mcg/kg/min every 5–10 minutes till desired level
• Use sedationscales.
• Consider dexmedetomidine 1mcg/kg followed by 0.2–0.7 mcg/kg/h
• Lorazepam bolus 2 mg followed by infusion 1 mg/h 
•Sedation score (opt any one score)
Target
•RASS=0 to –3 (Alert, calm, moderate sedation, movement or eye opening; no eye contact)
•SAS=3–4 (Sedated, difficult to arouse but awakens to verbal stimuli or gentle shaking)
•Ramsay=3 (Responds only to verbal commands)
Delirium
Pharmacological
treatment
Is patient delirious?
Nonpharmacological
treatment
•Dexmedetomidine 0.2–1.5 mcg/kg/h (Consider in patients failing spontaneous breathing trials secondary to agitation)
•Haloperidol 2.5–5 mg IV q 15 minutes till delirium present (suggested maximum35 mg/day) (Consider decreased dose in hypoactive delirium)
•Haloperidol 2.5–5 mg PO q 6 hours (Caution if baseline QTc > 440 ms)
•Quetiapine 50–200 mg PO q 12 hours (Consider if sedative properties desired)
•Risperidone 0.5–1 mg PO q 12 hours (Caution baseline QTc > 440 ms)
•Daily awakening trials
•Reorient patient regularly
•Early mobilization
•Promote effective sleep/awake cycles
•Timely removal of catheters/physical restraints
•Use of eyeglasses, magnifying lenses, and hearing aids
•Minimize noise/stimulation at night
•Minimize benzodiazepines for sedation
270 |
|
A.K. Mani et al. |
|
|
|
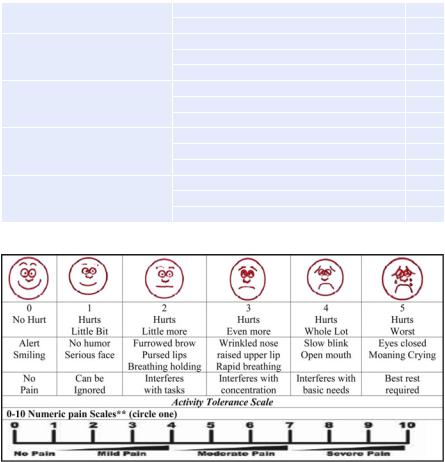
Table 33.3 The critical care pain observation tool (CPOT) (target 0 to 1) |
|
|
Facial expression |
Tense |
1 |
|
Grimacing |
2 |
Body movements |
Absence of movement or normal position |
0 |
|
Protection |
1 |
|
Restlessness |
2 |
Compliance with ventilator |
Tolerating the ventilator or movement |
0 |
(intubated patients) |
Coughing but tolerating |
1 |
|
Fighting the ventilator |
2 |
Vocalization (extubated patients) |
Talking in normal tone or no sound |
0 |
|
Sighing or moaning |
1 |
|
Crying out, sobbing |
2 |
Muscle tension |
Relaxed |
0 |
|
Tense, rigid |
1 |
|
Very tense or rigid |
2 |
Fig. 33.1 Wong-Baker FACES pain rating scale
Step 4: Assessment of pain, sedation, and delirium
¥Use of sedation and pain scales is recommended to titrate the appropriate level of analgesia and sedation.
¥There are many scores, but one score should be followed for uniformity in the ICU.
¥The following scales/scores may be used: A. Pain
¥A critical care pain observation tool (Table 33.3)
¥Self-reporting of pain
¥Wong-Baker FACES pain rating scale (Fig. 33.1)
¥0Ð10 numeric pain scales
33 Sedation and Analgesia |
|
271 |
|
|
|
Table 33.4 Richmond agitation-sedation scale (RASS) (target 0 to −3) |
|
|
Combative, violent, danger to staff |
+4 |
|
Pulls or removes tubes or catheters, aggressive |
+3 |
|
Frequent nonpurposeful movement, Þghts the ventilator |
+2 |
|
Anxious, apprehensive, but not aggressive |
+1 |
|
Alert and calm |
|
0 |
Awakens to voice (eye opening/contact) >10 s |
−1 |
|
Light sedation, brießy awakens to voice (eye opening/contact) <10 s |
−2 |
|
Moderate sedation, movement or eye opening (no eye contact) |
−3 |
|
Deep sedation, no response to voice, but movement or eye opening to physical stimulation |
−4 |
|
Unarousable, no response to voice or physical stimulation |
−5 |
|
Table 33.5 Riker sedation-agitation scale (SAS) (target sedation 3 to 4) |
|
|
Dangerous agitation, pulling the ETT, trying to remove catheters, climbing over bedrail, |
7 |
|
striking at staff, thrashing side to side |
|
|
Very agitated, requiring restraint and frequent verbal reminding of limits, biting the ETT |
6 |
|
Agitated, anxious or physically agitated, calms to verbal instructions |
5 |
|
Calm and cooperative easily arousable, follows commands |
4 |
|
Sedated, difÞcult to arouse but awakens to verbal stimuli or gentle shaking, follows simple |
3 |
|
commands but drifts off again |
|
|
Very sedated, arouses to physical stimuli but does not communicate or follow commands, |
2 |
|
may move spontaneously |
|
|
Unarousable, minimal or no response to noxious stimuli, does not communicate or follow |
1 |
|
commands |
|
|
Table 33.6 Ramsay sedation score (target sedation 3) |
|
|
Level/score |
Clinical description |
|
1Anxious and agitated
2Cooperative, oriented, tranquil
3Responds only to verbal commands
4Asleep with brisk response to light stimulation
5Asleep with sluggish response to stimulation
6Asleep without response to stimulation
B.Sedation
¥Richmond agitation-sedation scale (RASS) (Table 33.4)
¥Riker sedation-agitation scale (SAS) (Table 33.5)
¥Ramsay sedation score (Table 33.6)
C.Delirium
¥Confusion assessment method for the ICU
¥The intensive care delirium screening checklist
Step 5: Titrate sedation and analgesia
The titration of the sedative/analgesic dose to a deÞned endpoint is recommended.

272 |
A.K. Mani et al. |
|
|
Titrating infusions
Analgesia
The potential for opioid withdrawal should be considered for patients receiving high doses or 7 days of continuous therapy. Doses should be tapered systematically (i.e., 10Ð30% per day) to prevent withdrawal symptoms.
Sedation
Use RASS/SAS/Ramsay scores to evaluate. Titrate according to the target table.
¥Lorazepam/midazolam continuous infusion: Hold infusion until the patient reaches RASS goal, and then resume at one-half the previous rate. Titrate as per written orders.
¥Propofol continuous infusion: Decrease at the rate of 5Ð10 mcg/kg/min every 10 min until the patient reaches RASS goal. Titrate as per written orders.
¥Morphine/fentanyl continuous infusion: Hold until the patient reaches RASS goal, and then resume at one-half the previous rate. Titrate as per written orders.
Step 6: Daily awakening trial
¥Daily awakening trial is required to assess sedation and minimum effective dose for sedation.
¥It is recommended to couple spontaneous breathing trial (SBT) protocols with sedation protocols. Findings show that combined approach of spontaneous breathing trial and daily awakening results in less time on mechanical ventilation and less intensive care and the hospital stay.
¥Early exercise and mobilization (physical and occupational therapy) during periods of daily interruption of sedation has been shown to be safe and well tolerated and results in better functional outcomes at hospital discharge, a shorter duration of delirium, and more ventilator-free days compared with standard care.
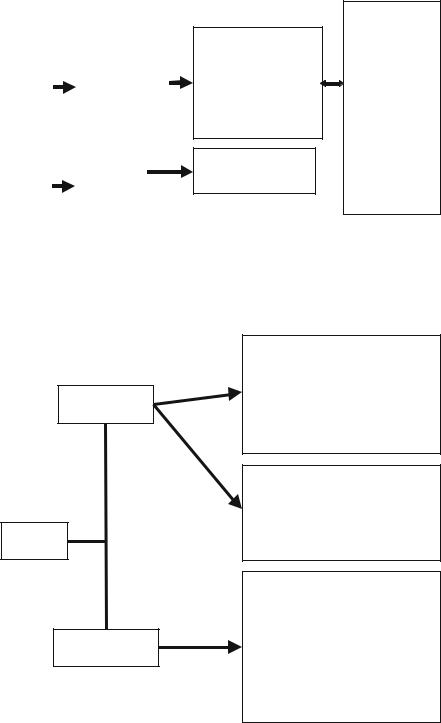
Assess for daily awakening Exclusions:
•Increased intracranial pressure
•Neuromuscular blockade
•Very high PEEP, FiO2
•CABG-immediate post-op
•Perform daily Awakening
•Wean/stop sedation
•Consider decreasing narcotics infusion by 25-50%
•Is patient awake and calm?
•Use sedation scale
•SAS 3–4 (sedated, difficult to arouse but awakens to verbal stimuli or gentle shaking, follows simple commands) or RASS 0 to – 1(alert and calm, awakens to voice (eye opening/contact) >10 seconds)
Yes
Spontaneous breathing trial
No
Restart sedation at previous dose (no rapid shallow breathing index)
