
- •ICU Protocols
- •Preface
- •Acknowledgments
- •Contents
- •Contributors
- •1: Airway Management
- •Suggested Reading
- •2: Acute Respiratory Failure
- •Suggested Reading
- •Suggested Reading
- •Website
- •4: Basic Mechanical Ventilation
- •Suggested Reading
- •Suggested Reading
- •Websites
- •Suggested Reading
- •Websites
- •7: Weaning
- •Suggested Reading
- •8: Massive Hemoptysis
- •Suggested Reading
- •9: Pulmonary Thromboembolism
- •Suggested Reading
- •Suggested Reading
- •Websites
- •11: Ventilator-Associated Pneumonia
- •Suggested Readings
- •12: Pleural Diseases
- •Suggested Reading
- •Websites
- •13: Sleep-Disordered Breathing
- •Suggested Reading
- •Websites
- •14: Oxygen Therapy
- •Suggested Reading
- •15: Pulse Oximetry and Capnography
- •Conclusion
- •Suggested Reading
- •Websites
- •16: Hemodynamic Monitoring
- •Suggested Reading
- •Websites
- •17: Echocardiography
- •Suggested Readings
- •Websites
- •Suggested Reading
- •Websites
- •19: Cardiorespiratory Arrest
- •Suggested Reading
- •Websites
- •20: Cardiogenic Shock
- •Suggested Reading
- •21: Acute Heart Failure
- •Suggested Reading
- •22: Cardiac Arrhythmias
- •Suggested Reading
- •Website
- •23: Acute Coronary Syndromes
- •Suggested Reading
- •Website
- •Suggested Reading
- •25: Aortic Dissection
- •Suggested Reading
- •26: Cerebrovascular Accident
- •Suggested Reading
- •Websites
- •27: Subarachnoid Hemorrhage
- •Suggested Reading
- •Websites
- •28: Status Epilepticus
- •Suggested Reading
- •29: Acute Flaccid Paralysis
- •Suggested Readings
- •30: Coma
- •Suggested Reading
- •Suggested Reading
- •Websites
- •32: Acute Febrile Encephalopathy
- •Suggested Reading
- •33: Sedation and Analgesia
- •Suggested Reading
- •Websites
- •34: Brain Death
- •Suggested Reading
- •Websites
- •35: Upper Gastrointestinal Bleeding
- •Suggested Reading
- •36: Lower Gastrointestinal Bleeding
- •Suggested Reading
- •37: Acute Diarrhea
- •Suggested Reading
- •38: Acute Abdominal Distension
- •Suggested Reading
- •39: Intra-abdominal Hypertension
- •Suggested Reading
- •Website
- •40: Acute Pancreatitis
- •Suggested Reading
- •Website
- •41: Acute Liver Failure
- •Suggested Reading
- •Suggested Reading
- •Websites
- •43: Nutrition Support
- •Suggested Reading
- •44: Acute Renal Failure
- •Suggested Reading
- •Websites
- •45: Renal Replacement Therapy
- •Suggested Reading
- •Website
- •46: Managing a Patient on Dialysis
- •Suggested Reading
- •Websites
- •47: Drug Dosing
- •Suggested Reading
- •Websites
- •48: General Measures of Infection Control
- •Suggested Reading
- •Websites
- •49: Antibiotic Stewardship
- •Suggested Reading
- •Website
- •50: Septic Shock
- •Suggested Reading
- •51: Severe Tropical Infections
- •Suggested Reading
- •Websites
- •52: New-Onset Fever
- •Suggested Reading
- •Websites
- •53: Fungal Infections
- •Suggested Reading
- •Suggested Reading
- •Website
- •55: Hyponatremia
- •Suggested Reading
- •56: Hypernatremia
- •Suggested Reading
- •57: Hypokalemia and Hyperkalemia
- •57.1 Hyperkalemia
- •Suggested Reading
- •Website
- •58: Arterial Blood Gases
- •Suggested Reading
- •Websites
- •59: Diabetic Emergencies
- •59.1 Hyperglycemic Emergencies
- •59.2 Hypoglycemia
- •Suggested Reading
- •60: Glycemic Control in the ICU
- •Suggested Reading
- •61: Transfusion Practices and Complications
- •Suggested Reading
- •Websites
- •Suggested Reading
- •Website
- •63: Onco-emergencies
- •63.1 Hypercalcemia
- •63.2 ECG Changes in Hypercalcemia
- •63.3 Superior Vena Cava Syndrome
- •63.4 Malignant Spinal Cord Compression
- •Suggested Reading
- •64: General Management of Trauma
- •Suggested Reading
- •65: Severe Head and Spinal Cord Injury
- •Suggested Reading
- •Websites
- •66: Torso Trauma
- •Suggested Reading
- •Websites
- •67: Burn Management
- •Suggested Reading
- •68: General Poisoning Management
- •Suggested Reading
- •69: Syndromic Approach to Poisoning
- •Suggested Reading
- •Websites
- •70: Drug Abuse
- •Suggested Reading
- •71: Snakebite
- •Suggested Reading
- •72: Heat Stroke and Hypothermia
- •72.1 Heat Stroke
- •72.2 Hypothermia
- •Suggested Reading
- •73: Jaundice in Pregnancy
- •Suggested Reading
- •Suggested Reading
- •75: Severe Preeclampsia
- •Suggested Reading
- •76: General Issues in Perioperative Care
- •Suggested Reading
- •Web Site
- •77.1 Cardiac Surgery
- •77.2 Thoracic Surgery
- •77.3 Neurosurgery
- •Suggested Reading
- •78: Initial Assessment and Resuscitation
- •Suggested Reading
- •79: Comprehensive ICU Care
- •Suggested Reading
- •Website
- •80: Quality Control
- •Suggested Reading
- •Websites
- •81: Ethical Principles in End-of-Life Care
- •Suggested Reading
- •82: ICU Organization and Training
- •Suggested Reading
- •Website
- •83: Transportation of Critically Ill Patients
- •83.1 Intrahospital Transport
- •83.2 Interhospital Transport
- •Suggested Reading
- •84: Scoring Systems
- •Suggested Reading
- •Websites
- •85: Mechanical Ventilation
- •Suggested Reading
- •86: Acute Severe Asthma
- •Suggested Reading
- •87: Status Epilepticus
- •Suggested Reading
- •88: Severe Sepsis and Septic Shock
- •Suggested Reading
- •89: Acute Intracranial Hypertension
- •Suggested Reading
- •90: Multiorgan Failure
- •90.1 Concurrent Management of Hepatic Dysfunction
- •Suggested Readings
- •91: Central Line Placement
- •Suggested Reading
- •92: Arterial Catheterization
- •Suggested Reading
- •93: Pulmonary Artery Catheterization
- •Suggested Reading
- •Website
- •Suggested Reading
- •95: Temporary Pacemaker Insertion
- •Suggested Reading
- •96: Percutaneous Tracheostomy
- •Suggested Reading
- •97: Thoracentesis
- •Suggested Reading
- •98: Chest Tube Placement
- •Suggested Reading
- •99: Pericardiocentesis
- •Suggested Reading
- •100: Lumbar Puncture
- •Suggested Reading
- •Website
- •101: Intra-aortic Balloon Pump
- •Suggested Reading
- •Appendices
- •Appendix A
- •Appendix B
- •Common ICU Formulae
- •Appendix C
- •Appendix D: Syllabus for ICU Training
- •Index

248 |
K. Kadapatti and S. Iyer |
|
|
Table 30.3 Coma mimics
Persistent vegetative state
Locked in syndrome
Akinetic mutism
Hypersomnia
Brain death
Generalized muscle weakness (snake bite, organophosphorus poisoning, Guillain–Barré syndrome, myasthenia gravis, severe hypokalemia, neuromuscular blockade)
Pseudocoma (malingering catatonia, conversion reaction, hysteria)
Step 6: Rule out coma mimics (Table 30.3)
• Some clinical entities may be confused with coma and need to be differentiated.
Step 7: Assess prognosis and communicate to the family
•This is dependent on the underlying cause. Reversible causes such as metabolic, toxic, and surgically amenable lesions have a better prognosis.
•Anoxic brain injury, diffuse cortical, or brainstem lesions carry a bad prognosis.
•Prognostication should be guarded initially in all cases of coma; frequent examination and time course of comatose state will ultimately dictate the prognosis.
Suggested Reading
1.Karanjia N, Geocadin RG. Post-cardiac arrest syndrome: update on brain injury management and prognostication. Curr Treat Options Neurol. 2011;13(2):191–203.
This article focuses on the neurological care of patients after they have been resuscitated from cardiac arrest. Maximizing neurological outcome after cardiac arrest requires attention to prevention of primary and secondary brain injury.
2.Geocadin RG, Eleff SM. Cardiac arrest resuscitation: neurologic prognostication and brain death. Curr Opin Crit Care. 2008;14(3):261–8.
Evidence-based tests of prognostication for neurological outcome after cardiac arrest are presented. A review of the practice of withdrawal of life-sustaining therapies and the diagnosis of brain death is also provided. The reader is cautioned that most prognostic studies do not include possible amelioration with the use of therapeutic hypothermia.
3.Wijdicks EF, Hijdra A, Young GB. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006; 67(2):203–10.
Pupillary light response, corneal reflexes, motor responses to pain, myoclonus status epilepticus, serum neuron-specific enolase, and somatosensory evoked potential studies can reliably assist in accurately predicting poor outcome in comatose patients after cardiopulmonary resuscitation for cardiac arrest.

Intracranial Pressure Monitoring |
31 |
and Management |
Rajagopal Senthilkumar and Nagarajan Ramakrishnan
A 30-year-old male with head injury was on a ventilator. He was withdrawing from painful stimulus. His pupillary responses were equal and brain CT scan showed bilateral frontal contusion and subarachnoid hemorrhage. His blood pressure (BP) was 100/60 mmHg, and SpO2 was 93% on 0.6 FiO2. His temperature was 100°F and blood sugar was 70 mg/dL. He had been nursed with the head elevated at 45°.
Increased intracranial pressure (ICP) should be suspected in all patients with altered mental state, especially due to an intracranial pathology. Prompt assessment and management of this problem prevents secondary brain injury.
Step 1: Initiate resuscitation
•If elevated ICP is suspected, care should be taken to minimize its rise during intubation through careful positioning and adequate sedation.
•Avoid hypercapnia as it raises ICP by causing vasodilation.
•Avoid succinylcholine during intubation as it may increase ICP.
•Pretreat with mannitol if pupils are unequal.
•Large shifts in blood pressure should be minimized, with particular care taken to avoid hypotension. Hypotension, especially in conjunction with hypoxemia, can induce reactive vasodilation and elevations in ICP.
•Vasopressors have been shown to be safe in most patients with intracranial hypertension and may be required to maintain cerebral perfusion pressure (CPP) of more than 50 mmHg.
R. Senthilkumar, M.D., E.D.I.C. (*) • N. Ramakrishnan, A.B.(I.M.), F.A.C.P. Department of Critical Care Medicine, Apollo Hospitals, Chennai, India e-mail: rskumar@vsnl.net
R. Chawla and S. Todi (eds.), ICU Protocols: A stepwise approach, |
249 |
DOI 10.1007/978-81-322-0535-7_31, © Springer India 2012 |
|
250 |
R. Senthilkumar and N. Ramakrishnan |
|
|
Step 2: Recognize features of increased ICP
•Raised ICP may present with symptoms of headache, altered level of consciousness, weakness of extremities, or as respiratory arrest. Careful clinical examination would reveal one or more of the following nonspecific signs. Frequent neurological examination is essential as these patients may deteriorate suddenly.
–Dilatation of ipsilateral or contralateral pupil
–Ptosis
–Hemiparesis
–Alteration of respiration
–Decerebrate posturing
–Bradycardia
–Hypertension
Step 3: Urgently manage increased ICP
•Urgent measures may need to be instituted prior to a more detailed workup (e.g., imaging or ICP monitoring) in a patient who presents acutely with history or examination findings suggestive of elevated ICP.
•Many of these situations will rely on clinical judgment, but the following combination of findings suggests the need for urgent intervention:
–A Glasgow Coma Scale (GCS) £8 in the absence of other systemic problems such as severe hypoxia, hypercapnia, hypotension, hypoglycemia, hypothermia, or intoxication to explain the mental state.
–In such patients
•Osmotic diuretics should be used urgently, 10–20% intravenous mannitol (1–1.5 g/kg)
•Head elevation to 30–45°
•Hyperventilation to a PCO2 of 26–30 mmHg
•In addition, standard resuscitation techniques should be instituted as soon as possible.
•Prolonged hyperventilation is contraindicated in the setting of traumatic brain injury and acute stroke as hypocapnia and respiratory alkalosis will cause cerebral vasoconstriction and worsen perfusion.
•Ventriculostomy is a rapid means of simultaneously diagnosing and treating elevated ICP (Fig. 31.3).
Step 4: Identify causes of raised ICP (Table 31.1)
•Brain is enclosed in a closed compartment formed of bony skull. It consists of three essential elements: brain matter (noncompressible), CSF, and blood (arteries and veins). Increase in any one of the elements will displace the others to keep ICP constant till a point when there will be an exponential rise of ICP. Displacement of brain will cause herniation syndromes.
Step 5: Initiate ICP monitoring
•An important early goal in management of the patient with presumed elevated ICP is placement of an ICP monitoring device.

31 Intracranial Pressure Monitoring and Management |
251 |
|
|
Table 31.1 Common reasons for raised intracranial lesions
1. Localized mass lesions
•Traumatic hematomas (extradural, subdural, and intracerebral)
•Abscess
•Neoplasms
•ICH and massive cerebral infarction 2. Impaired CSF circulation
•Obstructive and communicating hydrocephalus 3. Obstruction to venous outflow
•Cerebral venous thrombosis
•Depressed fractures overlying major venous sinuses 4. Diffuse brain edema
•Infections and inflammations (encephalitis, meningitis)
•Diffuse head injury
•Hepatic encephalopathy
•Water intoxication
•Near-drowning
•The only way to reliably determine cerebral perfusion pressure (CPP defined as the difference between mean arterial pressure [MAP] and ICP) is to continuously monitor both ICP and BP.
•Normal ICP is below 20 mmHg.
•CPP should be kept between 50 and 70 mmHg in patients with elevated ICP in an attempt to avoid hypoperfusion and ischemic injury.
•Cerebral autoregulation maintains cerebral blood flow (CBF) by altering cerebral arteriolar diameter in response to changing CPP. Increase in CPP constricts the blood vessel, and decrease in CPP dilates the arterioles. Thus, CPP is a useful surrogate for CBF. In the injured brain, this autoregulation is lost, so CPP should be closely monitored and kept in a safe zone by monitoring ICP.
•Indications of ICP monitoring in severe head injury:
–Comatose patients with Glasgow coma score (GCS) of 3–8, and with abnormal cranial findings on computed tomographic (CT) scan
–Comatose patients with normal CT scans and more than 40 years of age
–Unilateral or bilateral motor posturing and systolic blood pressure (SBP) of less than 90 mmHg
•ICP monitoring is not widely practiced for fear of risk of infection and absence of definite outcome data on reduction of mortality.
•There are four main anatomical sites used in the clinical measurement of ICP: intraventricular, intraparenchymal, subarachnoid, and epidural.
•Intraventricular monitors are considered the gold standard of ICP monitoring catheters. They are surgically placed into the ventricular system and affixed to a drainage bag and pressure transducer with a three-way stopcock.
•Intraventricular monitoring has the advantage of accuracy, simplicity of measurement, and the unique characteristic of allowing for treatment of some causes

252 |
R. Senthilkumar and N. Ramakrishnan |
|
|
of elevated ICP via drainage of cerebrospinal fluid (CSF). The primary disadvantage is infection, which may occur in up to 20% of patients. This risk increases the longer a device is in place. A further disadvantage of intraventricular systems includes a small (~2%) risk of hemorrhage during placement, which is increased in coagulopathic patients. In addition, it may be technically difficult to place an intraventricular drain into a small ventricle, particularly in the setting of trauma and cerebral edema complicated by ventricular compression.
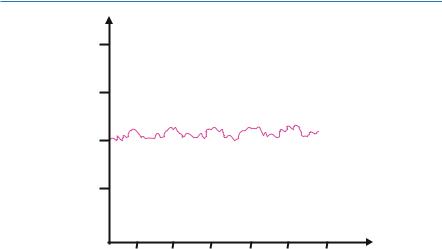
Step 6: Analyze ICP waveform (Figs. 31.1 and 31.2)
•ICP is not a static value; it exhibits cyclic variation based on the superimposed effects of cardiac contraction, respiration, and intracranial compliance.
•Under normal physiological conditions, the amplitude of the waveform is often small, with B waves related to respiration and smaller C waves related to the cardiac cycle.
•Pathological A waves (also called plateau waves) are abrupt, marked elevations in ICP of 50–100 mmHg, which usually last for minutes to hours.
•The presence of A waves signifies a loss of intracranial compliance and heralds imminent decompensation of autoregulatory mechanisms and needs urgent intervention to lower ICP.
|
40 |
Hg) |
20 |
pressure(mm |
|
|
30 |
Intracranial |
10 |
|
|
|
0 |
10 |
20 |
30 |
40 |
50 |
60 |
Time (minutes)
Fig. 31.1 A waves
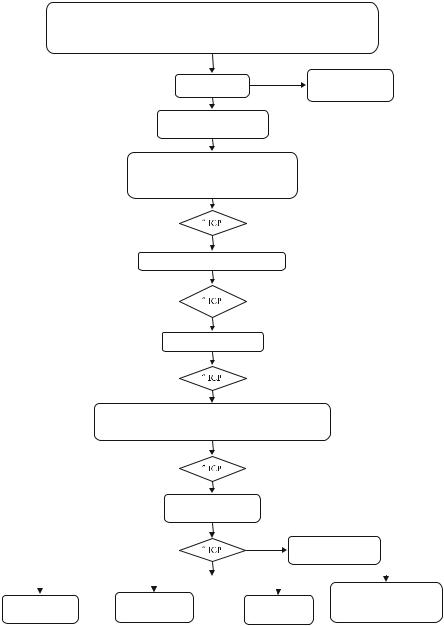
Step 7: Start specific management of increased ICP (Fig. 31.3)
Intracranial hypertension (ICH) is a medical emergency. The best therapy for ICH is resolution of the proximate cause of elevated ICP. Examples include the evacuation of a blood clot, resection of a tumor, CSF diversion in the setting of hydrocephalus, or treatment of an underlying metabolic disorder. Measures to lower ICP
31 Intracranial Pressure Monitoring and Management |
253 |
|
|
40 |
|
Hg) |
20 |
|
pressure(mm |
|
|
|
30 |
|
Intracranial |
10 |
|
|
|
|
|
0 |
|
1 2 3 4 5 6
Time (minutes)
Fig. 31.2 B waves
are generally applicable to all patients with suspected ICH. Some measures (particularly glucocorticoids) are reserved for specific causes of ICH.
•Mannitol
–Osmotic diuretics reduce the brain volume by drawing free water out of the tissue and into the circulation, where it is excreted by the kidneys, thus dehydrating brain parenchyma.
–The most commonly used agent is 20% solution of mannitol given as a bolus of 1 g/kg.
–Repeated dosing can be given at 0.25–0.5 g/kg as needed, generally every 6–8 h.
–Use of any osmotic agent should be carefully evaluated in patients with renal and cardiac insufficiency.
–Useful parameters to monitor in the setting of mannitol therapy include serum sodium, serum osmolality, and renal function.
–Concerned findings associated with the use of mannitol include serum sodium of more than 150 mEq, serum osmolality of more than 320 mOsm, or rising blood, urea, and creatinine suggestive of evolving acute tubular necrosis (ATN).
–Mannitol can lower systemic BP, necessitating careful use if associated with a fall in CPP.
–It can cause massive diuresis and loss of potassium, magnesium, and phosphorus.
–In patients on mannitol therapy, euvolemia should be maintained by replacing volume loss with normal saline and additive electrolytes.
–Measuring osmolar gap (measured—calculated serum osmolality) may be
useful in titrating mannitol therapy and should be kept below 18.
254 |
R. Senthilkumar and N. Ramakrishnan |
|
|
Initial resuscitation using ATLS guidelines o Airway – Secure airway using neuroprotective methods
oBreathing –Avoid hypoxia and hypercarbia. Goals PaO2> 60 mm Hg, PaCO2 35 – 40 mm Hg
CT scan of head
Surgical evacuation of mass lesion if indicated
ICP monitor
Maintain CPP > 40-65 mm Hg
Primary interventions for TBI patients o Elevate head of bed to 30
o Avoid hyperthermia. Maintain T < 38.0°C o Provide adequate sedation and analgesia
CSF drainage through ventriculostomy
Neuromuscular blockade
Hyperosmolar therapy
1.Mannitol-Maintain serum Osm < 320 mOsm/L
2.Hypertonic saline (3% NaCl) -Maintain serum Osm <360 mOsm/L
Hyperventilation maintain
PaCO3 30-35 mm Hg
|
|
|
|
|
Repeat head CT/surgery |
||
|
|
|
|
|
if indicated |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Lumbar drain with functional |
|
|
|
Hypothermia goal |
|
|
|||
High dose |
Decompressive |
ventricular drain, open cisterns, |
|||||
barbiturate therapy |
temperature 32-34°C |
craniectomy |
no signs of mass effect |
||||
Fig. 31.3 Raised ICP Management
31 Intracranial Pressure Monitoring and Management |
255 |
|
|
•Loop diuretics
–Furosemide, 0.5–1.0 mg/kg intravenously, may be given with mannitol to potentiate its effect. However, this effect can also exacerbate dehydration and hypokalemia.
•Hypertonic saline
–Hypertonic saline in bolus doses may acutely lower ICP.
–Advantages of hypertonic saline are its use in hypotensive patients, reduced potential to cause renal damage, and less hyponatremia.
–The volume and tonicity of saline (3–23.4%) used in these reports have varied widely.
–Use of the central line is recommended for 23% saline to prevent venous thrombosis.
–In patients without central venous access, continuous infusion of hypertonic saline (1.25–3%) may help to keep serum osmolality elevated.
–Target the serum sodium level of 150–160 mEq/L.
–Weaning from osmotherapy should be gentle as sharp decrease in serum sodium may cause cerebral edema. Every day, 5–8 mEq decrease in serum sodium is generally recommended.
•Glucocorticoids
–In general, glucocorticoids are not considered to be useful in the management of increased ICP due to cerebral infarction or intracranial hemorrhage.
–In contrast, glucocorticoids may have a role in the setting of intracranial hypertension caused by brain tumors and CNS infections.
•Hyperventilation
–The use of mechanical ventilation to lower PaCO2 to 26–30 mmHg has been shown to rapidly reduce ICP through vasoconstriction and a decrease in the volume of intracranial blood.
–The effect of hyperventilation on ICP is short-lived (1–24 h).
–Therapeutic hyperventilation may be considered as an urgent intervention when elevated ICP complicates cerebral edema, intracranial hemorrhage, and tumor.
–Hyperventilation should not be used on a chronic basis, regardless of the cause of ICH.
–Hyperventilation should be minimized in patients with traumatic brain injury or acute stroke. In these settings, vasoconstriction may cause a critical decrease in local cerebral perfusion and worsen neurological injury, particularly in the first 24–48 h.
–This might be used as a temporizing measure for patients awaiting a definitive therapy like surgical evacuation of a cerebral clot or tumor.
•Barbiturates
–The use of barbiturates is predicated on their ability to reduce brain metabolism and cerebral blood flow, thus lowering ICP and exerting a neuroprotective effect. However, the therapeutic value of this remains unclear.
–Pentobarbital is generally used, with a loading dose of 5–20 mg/kg as a bolus, followed by 1–4 mg/kg/h.
256 |
R. Senthilkumar and N. Ramakrishnan |
|
|
–Treatment should be assessed based on ICP, CPP, and the presence of unacceptable side effects.
–Continuous electroencephalogram (EEG) monitoring is generally recommended with EEG burst suppression as an indication of maximal dosing.
•Glycerol and urea
–These were used historically to control ICP via osmoregulation.
–The use of these agents has decreased because equilibration between brain and plasma levels occurs more quickly than with mannitol.
–Furthermore, glycerol has been shown to have a significant rebound effect and to be less effective in ICP control.
•Therapeutic hypothermia
–It is not currently recommended as a standard treatment for increased intracranial pressure.
•Neuromuscular paralysis
–This should generally be avoided unless the patient has refractory rise of ICP and is being closely monitored.
•Removal of CSF
–When hydrocephalus is identified, a ventriculostomy should be inserted.
–Rapid aspiration of CSF should be avoided because it may lead to obstruction of the catheter opening by brain tissue.
–In patients with aneurysmal subarachnoid hemorrhage, abrupt lowering of the pressure differential across the aneurysm dome can precipitate recurrent hemorrhage.
–CSF should be removed at a rate of approximately 1–2 mL/min, for 2–3 min at a time, with intervals of 2–3 min in between until a satisfactory ICP has been achieved (ICP < 20 mmHg) or until CSF is no longer easily obtained.
–Slow removal can also be accomplished by passive gravitational drainage through the ventriculostomy, with positioning of the bag raised at the desired level of intracranial pressure.
–A lumbar drain is generally contraindicated in the setting of high ICP due to the risk of transtentorial herniation.
•Decompressive craniectomy
–Decompressive craniectomy removes the rigid confines of the bony skull, increasing the potential volume of the intracranial contents.
–It has been demonstrated that in patients with elevated ICP, craniectomy alone lowers ICP up to 15%.
–Opening the dura in addition to the bony skull results in an average decrease in ICP of 70%.
–A recent study (DECRA) has shown the worse 6-month outcome with this procedure in the severe traumatic brain injury patient.
Step 8: Start general measures of management
•Fluid management
–In general, patients with elevated ICP do not need to be severely fluid restricted.
31 Intracranial Pressure Monitoring and Management |
257 |
|
|
–Patients should be kept euvolemic and normoto hyperosmolar.
–This can be achieved by avoiding free water and employing only isotonic fluids (such as 0.9% saline).
–Serum osmolality should be kept to more than 280 mOsm/L and often is kept in the 295–305 mOsm/L range.
•Sedation and pain management
–Keeping patients appropriately sedated and pain-free can decrease ICP by reducing metabolic demand, ventilator asynchrony, venous congestion, and the sympathetic responses of hypertension and tachycardia.
–Propofol has been utilized to good effect in this setting, as it is easily titrated and has a short half-life, thus permitting frequent neurological reassessment. Newer agents like dexmedetomidine may also be used for this purpose.
–Fentanyl should be used cautiously as it may raise ICP.
–Agitated patients may be sedated with short-acting benzodiazepines.
–The use of end-tidal CO2 should be performed frequently in sedated patients with increased ICP to maintain normocarbia. Trending upward of end-tidal CO2 should warrant urgent attention.
•Blood pressure control
–In general, BP should be sufficient to maintain CPP of more than 50 mmHg.
–Vasopressors can be used safely without further increasing ICP. This is particularly relevant in the setting of sedation, when drug-induced hypotension can occur.
–Hypertension should generally only be treated when CPP is more than 120 mmHg and ICP is more than 20 mmHg.
–Labetalol and nicardipine are the ideal choice.
–Nitrates should be avoided as they increase CBF.
•Position
–Patients with elevated ICP should be positioned to maximize venous outflow from the head and are traditionally managed with the head elevated above the heart (usually 30°).
–Important maneuvers include reducing excessive flexion or rotation of the neck, avoiding restrictive neck taping, and minimizing stimuli that could induce Valsalva responses, such as endotracheal suctioning.
•Temperature control
–Fever increases brain metabolism and has been demonstrated to increase brain injury.
–Aggressive treatment of fever, including acetaminophen and mechanical cooling, is recommended in patients with increased ICP.
•Anticonvulsant therapy
–Seizures can complicate and contribute to elevated ICP.
–Anticonvulsant therapy should be instituted if seizures are suspected.
–Prophylactic treatment may be warranted in some cases.
–There are no clear guidelines for prophylactic antiepileptic, but examples include high-risk mass lesions, such as those within supratentorial cortical locations, or lesions adjacent to the cortex, such as subdural hematomas or subarachnoid hemorrhage.
