
- •ICU Protocols
- •Preface
- •Acknowledgments
- •Contents
- •Contributors
- •1: Airway Management
- •Suggested Reading
- •2: Acute Respiratory Failure
- •Suggested Reading
- •Suggested Reading
- •Website
- •4: Basic Mechanical Ventilation
- •Suggested Reading
- •Suggested Reading
- •Websites
- •Suggested Reading
- •Websites
- •7: Weaning
- •Suggested Reading
- •8: Massive Hemoptysis
- •Suggested Reading
- •9: Pulmonary Thromboembolism
- •Suggested Reading
- •Suggested Reading
- •Websites
- •11: Ventilator-Associated Pneumonia
- •Suggested Readings
- •12: Pleural Diseases
- •Suggested Reading
- •Websites
- •13: Sleep-Disordered Breathing
- •Suggested Reading
- •Websites
- •14: Oxygen Therapy
- •Suggested Reading
- •15: Pulse Oximetry and Capnography
- •Conclusion
- •Suggested Reading
- •Websites
- •16: Hemodynamic Monitoring
- •Suggested Reading
- •Websites
- •17: Echocardiography
- •Suggested Readings
- •Websites
- •Suggested Reading
- •Websites
- •19: Cardiorespiratory Arrest
- •Suggested Reading
- •Websites
- •20: Cardiogenic Shock
- •Suggested Reading
- •21: Acute Heart Failure
- •Suggested Reading
- •22: Cardiac Arrhythmias
- •Suggested Reading
- •Website
- •23: Acute Coronary Syndromes
- •Suggested Reading
- •Website
- •Suggested Reading
- •25: Aortic Dissection
- •Suggested Reading
- •26: Cerebrovascular Accident
- •Suggested Reading
- •Websites
- •27: Subarachnoid Hemorrhage
- •Suggested Reading
- •Websites
- •28: Status Epilepticus
- •Suggested Reading
- •29: Acute Flaccid Paralysis
- •Suggested Readings
- •30: Coma
- •Suggested Reading
- •Suggested Reading
- •Websites
- •32: Acute Febrile Encephalopathy
- •Suggested Reading
- •33: Sedation and Analgesia
- •Suggested Reading
- •Websites
- •34: Brain Death
- •Suggested Reading
- •Websites
- •35: Upper Gastrointestinal Bleeding
- •Suggested Reading
- •36: Lower Gastrointestinal Bleeding
- •Suggested Reading
- •37: Acute Diarrhea
- •Suggested Reading
- •38: Acute Abdominal Distension
- •Suggested Reading
- •39: Intra-abdominal Hypertension
- •Suggested Reading
- •Website
- •40: Acute Pancreatitis
- •Suggested Reading
- •Website
- •41: Acute Liver Failure
- •Suggested Reading
- •Suggested Reading
- •Websites
- •43: Nutrition Support
- •Suggested Reading
- •44: Acute Renal Failure
- •Suggested Reading
- •Websites
- •45: Renal Replacement Therapy
- •Suggested Reading
- •Website
- •46: Managing a Patient on Dialysis
- •Suggested Reading
- •Websites
- •47: Drug Dosing
- •Suggested Reading
- •Websites
- •48: General Measures of Infection Control
- •Suggested Reading
- •Websites
- •49: Antibiotic Stewardship
- •Suggested Reading
- •Website
- •50: Septic Shock
- •Suggested Reading
- •51: Severe Tropical Infections
- •Suggested Reading
- •Websites
- •52: New-Onset Fever
- •Suggested Reading
- •Websites
- •53: Fungal Infections
- •Suggested Reading
- •Suggested Reading
- •Website
- •55: Hyponatremia
- •Suggested Reading
- •56: Hypernatremia
- •Suggested Reading
- •57: Hypokalemia and Hyperkalemia
- •57.1 Hyperkalemia
- •Suggested Reading
- •Website
- •58: Arterial Blood Gases
- •Suggested Reading
- •Websites
- •59: Diabetic Emergencies
- •59.1 Hyperglycemic Emergencies
- •59.2 Hypoglycemia
- •Suggested Reading
- •60: Glycemic Control in the ICU
- •Suggested Reading
- •61: Transfusion Practices and Complications
- •Suggested Reading
- •Websites
- •Suggested Reading
- •Website
- •63: Onco-emergencies
- •63.1 Hypercalcemia
- •63.2 ECG Changes in Hypercalcemia
- •63.3 Superior Vena Cava Syndrome
- •63.4 Malignant Spinal Cord Compression
- •Suggested Reading
- •64: General Management of Trauma
- •Suggested Reading
- •65: Severe Head and Spinal Cord Injury
- •Suggested Reading
- •Websites
- •66: Torso Trauma
- •Suggested Reading
- •Websites
- •67: Burn Management
- •Suggested Reading
- •68: General Poisoning Management
- •Suggested Reading
- •69: Syndromic Approach to Poisoning
- •Suggested Reading
- •Websites
- •70: Drug Abuse
- •Suggested Reading
- •71: Snakebite
- •Suggested Reading
- •72: Heat Stroke and Hypothermia
- •72.1 Heat Stroke
- •72.2 Hypothermia
- •Suggested Reading
- •73: Jaundice in Pregnancy
- •Suggested Reading
- •Suggested Reading
- •75: Severe Preeclampsia
- •Suggested Reading
- •76: General Issues in Perioperative Care
- •Suggested Reading
- •Web Site
- •77.1 Cardiac Surgery
- •77.2 Thoracic Surgery
- •77.3 Neurosurgery
- •Suggested Reading
- •78: Initial Assessment and Resuscitation
- •Suggested Reading
- •79: Comprehensive ICU Care
- •Suggested Reading
- •Website
- •80: Quality Control
- •Suggested Reading
- •Websites
- •81: Ethical Principles in End-of-Life Care
- •Suggested Reading
- •82: ICU Organization and Training
- •Suggested Reading
- •Website
- •83: Transportation of Critically Ill Patients
- •83.1 Intrahospital Transport
- •83.2 Interhospital Transport
- •Suggested Reading
- •84: Scoring Systems
- •Suggested Reading
- •Websites
- •85: Mechanical Ventilation
- •Suggested Reading
- •86: Acute Severe Asthma
- •Suggested Reading
- •87: Status Epilepticus
- •Suggested Reading
- •88: Severe Sepsis and Septic Shock
- •Suggested Reading
- •89: Acute Intracranial Hypertension
- •Suggested Reading
- •90: Multiorgan Failure
- •90.1 Concurrent Management of Hepatic Dysfunction
- •Suggested Readings
- •91: Central Line Placement
- •Suggested Reading
- •92: Arterial Catheterization
- •Suggested Reading
- •93: Pulmonary Artery Catheterization
- •Suggested Reading
- •Website
- •Suggested Reading
- •95: Temporary Pacemaker Insertion
- •Suggested Reading
- •96: Percutaneous Tracheostomy
- •Suggested Reading
- •97: Thoracentesis
- •Suggested Reading
- •98: Chest Tube Placement
- •Suggested Reading
- •99: Pericardiocentesis
- •Suggested Reading
- •100: Lumbar Puncture
- •Suggested Reading
- •Website
- •101: Intra-aortic Balloon Pump
- •Suggested Reading
- •Appendices
- •Appendix A
- •Appendix B
- •Common ICU Formulae
- •Appendix C
- •Appendix D: Syllabus for ICU Training
- •Index

Arterial Blood Gases |
58 |
|
|
Rahul Pandit |
|
A 45-year-old alcoholic male patient was admitted to the hospital for 2 weeks. He was being treated for pyogenic lung abscess. He seemed to be improving but became unwell again. A blood gas analysis showed pH 7.31, PaCO2 30 mmHg, PaO2 106 mmHg (0.3 FiO2), HCO3− 14 mmol/L, standard base excess (SBE) 15 mmol/L, Na+ 131 mmol/L, K+ 5 mmol/L, Cl− 96 mmol/L, osmolar gap 8 mmol/Kg, lactate 2 mmol/L, and albumin 3 g/dL.
Arterial blood gas analysis is an essential component of diagnosing and managing critically ill patients in the ICU. The proper understanding and application of the concepts of acid–base balance will help the clinician to follow the progress of the patient and also to evaluate the effectiveness of the treatment provided to them.
Step 1: Take an arterial blood sample
•If possible, take an arterial blood gas (ABG) sample at room air and start oxygen supplementation immediately.
•The radial artery is preferred for collecting the sample.
•Prefer to use 22-gauge needle.
•Avoid air bubbles.
•Cool the sample immediately. A. Potential sampling error.
•Air contamination—spurious increase in PO2.
•Duration of exposure is more important than volume of air bubbles.
•Expel air immediately.
•Discard the sample if froth is present.
R. Pandit, M.D., F.C.I.C.M. (*)
Department of Critical Care, Seven Hills Hospital, Mumbai, India e-mail: dr_rapandit@yahoo.com
R. Chawla and S. Todi (eds.), ICU Protocols: A stepwise approach, |
455 |
DOI 10.1007/978-81-322-0535-7_58, © Springer India 2012 |
|

456 |
R. Pandit |
|
|
B. Venous sample—absence of flash of blood on entry into the vessel and pulsations during syringe filling and absence of autofilling of the syringe.
•Venous admixture.
•Cross-check with pulse oximetry and clinical status.
Anticoagulant effects: Dilution error—drop in PCO2 and PO2. pH usually remains unchanged.
D.Metabolism: Blood cells consume O2, produce CO2, and lower pH. Magnitude of changes depends on the initial level.
Step 2: Take detailed history and do proper clinical examination
•Very often, it is the presenting symptom or signs which are a clue to the interpretation of the acid–base status.
•For example in a patient with vomiting, the primary acid–base problem could be metabolic alkalosis (due to loss of hydrochloric acid) as opposed to someone with diarrhea, whose primary problem could be metabolic acidosis (due to loss of bicarbonate ions).
•The important aspect to remember is that it is the underlying disorder of the patient which determines the acid–base status and not just the pH of the blood.
•A stepwise approach helps to interpret ABG correctly.
•Interpretation of serum electrolytes is also important for an accurate estimation of mixed acid–base disorders.
Step 3: Know the normal values
|
Normal range |
For calculation |
pH |
7.34–7.45 |
7.4 |
PCO2 |
35–45 |
40 |
HCO3 |
22–26 |
24 |
PO2 |
>80 |
>95 |
Step 4: Assess oxygenation (Ref Appendix 2)
•Look at oxygenation (PaO2 and SaO2).
•Look at the PaO2/FiO2 ratio.
–Normally, the ratio is around 1:400–1:500 given the fact that at 0.21 FiO2, the PaO2 is approximately 100 mmHg.
–Less than 1:400—suggestive of V–Q mismatch or diffusion defect or intracardiac shunt.
–Less than 300 with bilateral lung infiltrate in chest skiagram—ARDS.
•A-a gradient
–A-a gradient = PAO2 − PaO2
Here, PAO2 is alveolar PO2 (calculated from the alveolar gas equation) and PaO2
is arterial PO2 (measured in arterial blood A-a gradient). |
|
|
|
– |
In general, the A-a gradient can be calculated by: |
/ 0.8) |
|
– |
A − a gradient = FiO2 × (Patm − PH2 O)− (PaCO2 |
− PaO2 |
|
|
|
|
|
58 Arterial Blood Gases |
457 |
|
|
On room air and at sea level, the FiO2 is 0.21, the Patm is 760 mmHg, and PH2O is 47 mmHg.
– On room air, PAO2 can be calculated by:
150 − PaCO2 / 0.8
•Normal A-a gradient in a 20-year-old person is 5 mmHg, which increases to 10 mmHg in a 35-year-old person. If A-a gradient is 20 mmHg at any age, it is abnormal. If FiO2 is above 0.21, it is unreliable.
Step 5: Assess acid–base disorder (Ref Appendix 2)
I. Look at the pH—is there acidemia or alkalemia?
A normal pH would suggest a mixed disorder or a normal acid–base status.
II.Check CO2 and HCO3− to determine whether the primary problem is metabolic or respiratory in origin.
III.If the primary disorder is respiratory, determine whether it is an acute disorder or a chronic disorder.
IV. Apply the rules of compensation to know if it is a simple or a mixed disorder. V. Mind the gaps—anion gap, delta gap, and osmolar gap.
I.Look at the pH
The pH is actually the −log [H+]. By altering either the PCO2 or the HCO3−, [H+] will change, and so will pH.
•An acidemia (low pH) can result from either a low HCO3 or a high CO2.
•An alkalemia (high pH) can result from either a high HCO3 or a low CO2.
II. Look at the CO2 and HCO3− to determine if the primary problem is metabolic or respiratory in origin
The primary acid–base disturbances include the following:
•Low HCO3—metabolic acidosis
•High HCO3—metabolic alkalosis
•High PCO2—respiratory acidosis
•Low PCO2—respiratory alkalosis A. Metabolic acidosis
•Metabolic acidosis results from a primary decrease in plasma [HCO3−].
•It is due to either an excretion of bicarbonate-containing fluids or by utilization of bicarbonate.
•It is very important to calculate the anion gap (AG) if the primary disorder is metabolic acidosis.
•AG = Na+ − (Cl− + HCO3−); the normal AG is 12 ±2 mEq/L.
•A higher gap usually denotes the presence of unmeasured anions in the body (Table 58.1).
•In non-AG metabolic acidosis, the bicarbonate losses are accompanied by cation loss, hence no change in AG (Table 58.2).

458 |
R. Pandit |
|
|
Table 58.1 Causes of a raised AG metabolic acidosis (MUDPILERS)
M Methanol
U Uremia (chronic)
DDiabetic ketoacidosis
PParaldehyde
IIsoniazid, iron
LLactate
EEthanol, ethylene glycol
RRhabdomyolysis/renal failure
SSalicylate
Table 58.2 Causes of a non-AG metabolic acidosis (HARDUP)
H Hyperalimentation
A Acetazolamide
R Renal tubular acidosis
D Diarrhea
U Uremia (acute)
P Post ventilation hypocapnia
•Remember to correct AG for hypoalbuminemia, which is very common in ICU patients. For this, for every 1 g% drop in albumin below 4 g%, add 2–3 to the calculated gap.
•Check urinary AG in non-AG metabolic acidosis (U Na+ +U K−U Cl)
–Normal—negative
–Nonrenal loss of bicarbonate (diarrhea)—negative
–Renal loss of bicarbonate or decrease H+ excretion (renal tubular acidosis)—positive
B.Metabolic alkalosis (high HCO3)
•Metabolic alkalosis reflects an increase in plasma [HCO3−].
•It is due to either gain of HCO3− or extracellular volume contraction.
•It can be classified into saline responsive or nonresponsive. For this, spot urinary chloride can be checked.
•More than 20 mEq/L urinary chloride is saline unresponsive (Table 58.3), and less than 20 mEq/L urinary chloride is saline responsive (Table 58.4).
C.Respiratory acidosis (high PCO2)
•Respiratory acidosis is due to a primary rise in CO2.
•Hypercapnia almost always results from alveolar hypoventilation due to one of the following causes:
1. Respiratory center depression
2. Neuromuscular disorders
3. Upper airway obstruction
4. Pulmonary disease
58 Arterial Blood Gases |
459 |
|
|
Table 58.3 Urine Cl− more than 20 mEq/L (usually saline unresponsive)
Primary hyperaldosteronism
Cushing’s syndrome, ectopic ACTH
Exogenous steroids, licorice ingestion, tobacco chewing
Adrenal 11 or 17 OH defects
Liddle’s syndrome
Bartter’s syndrome
K+ and Mg2+ deficiency
Milk-alkali syndrome
Hypercalcemia with secondary hypoparathyroidism
Table 58.4 Urine Cl− less than 20 mEq/L (usually saline responsive)
Vomiting, nasogastric suctioning
Chloride-wasting diarrhea
Villous adenoma of colon
Posthypercapnia
Poorly reabsorbed anions like carbenicillin
Diuretic therapy
D.Respiratory alkalosis (low PCO2)
•A respiratory alkalosis is due to decrease in PCO2.
•It results from hyperventilation leading to decrease in CO2.
Causes of respiratory alkalosis
– Hypoxemia from any cause
– Respiratory center stimulation
– Mechanical hyperventilation
– Sepsis, pain
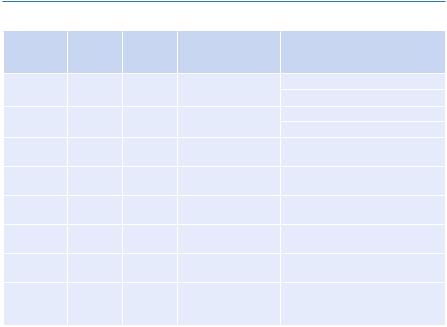
III.If the primary disorder is respiratory, determine whether it is an acute disorder or a chronic disorder
You must also take into consideration the patient’s history while interpreting ABG. However, the following formulae help in this:
–Normal pH is 7.4
–Calculate the change in pH (from 7.4)
A. In acute respiratory disorder (acidosis or alkalosis)
Change in pH = 0.008 × (PaCO2 − 40) Expected pH = 7.4 ± change in pH
B. In chronic respiratory disorder (acidosis or alkalosis)
Change in pH = 0.003 × (PaCO2 − 40) Expected pH = 7.4 ± change in pH
–Compare the pH on ABG
• If pH on ABG is close to A, it is an acute disorder
• If pH on ABG is close to B, it is a chronic disorder
460 R. Pandit
IV. CO |
2 |
and HCO |
|
− compensatory mechanism |
|
|
|||
|
|
Initial |
3 |
Compen- |
|
|
|
||
|
|
|
|
|
|
|
|
||
Primary |
|
|
chemical |
satory |
Compensatory |
|
|
||
disorder |
|
|
change |
|
|
response |
mechanism |
Expected level of compensation |
|
Metabolic |
|
↓ HCO3− |
|
↓ PCO2 |
Hyperventilation |
PCO2 = (1.5 × [HCO3−]) + 8 ± 2 |
|||
acidosis |
|
|
↑ HCO3− |
|
↑ PCO2 |
|
PCO2 = last two digits of pH |
||
Metabolic |
|
|
Hypoventilation |
PCO2 = (0.7 × [HCO3−]) + 21 ± 2 |
|||||
alkalosis |
|
|
↑ PCO2 |
|
|
↑ HCO3− |
|
PCO2 = last two digits of PH |
|
Respiratory |
|
|
|
|
|
||||
acidosis |
|
|
|
|
|
|
|
|
|
Acute |
|
|
|
|
|
|
Buffering—rule of 1 |
↑ [HCO3−])] = 1 mEq/L for every |
|
|
|
|
|
|
|
|
|
10 mmHg delta PCO2 |
|
Chronic |
|
|
|
|
|
|
Generation of new |
↑ [HCO3−]) = 3 mEq/L for every |
|
|
|
|
|
|
|
|
HCO −—rule of 3 |
10 mmHg delta PCO |
2 |
|
|
|
↓ PCO2 |
|
|
↓ HCO3 |
3 |
|
|
Respiratory |
|
|
|
|
|
||||
alkalosis |
|
|
|
|
|
|
|
|
|
Acute |
|
|
|
|
|
|
Buffering—rule of 2 |
↓ [HCO3−]) = 2 mEq/L for every |
|
|
|
|
|
|
|
|
|
10 mmHg delta PCO2 |
|
Chronic |
|
|
|
|
|
|
Decreased |
↓ [HCO3−]) = 4 mEq/L for every |
|
|
|
|
|
|
|
|
reabsorption of |
10 mmHg delta PCO2 |
|
|
|
|
|
|
|
|
HCO −—rule of 4 |
|
|
|
|
|
|
|
|
|
3 |
|
|
V.Mind the gaps
A1. Calculate AG in case of metabolic acidosis.
High denotes raised AG metabolic acidosis, and normal or narrow denotes nonAG acidosis.
A2. Calculate adjusted AG.
Adjusted AG = calculated AG + 2.5 × (4 − serum albumin in gm%)
B. In less obvious cases, the coexistence of two metabolic acid–base disorders may be apparent by calculating the difference between the change in AG
(delta AG) and the change in serum HCO3− (delta HCO3−). This calculation is called the bicarbonate gap or the delta gap:
•Delta gap = delta AG − delta HCO3−
•Where delta AG = patient’s AG − 12 mEq/L{normal AG}
•Delta HCO3− = 24 mEq/L{normal HCO3−} − patient’s HCO3−
•Normally, the delta gap is zero if there is only AG acidosis. A positive raised delta gap or a decreased delta gap denotes presence of mixed lesion.
•A positive delta gap of more than 6 mEq/L is suggestive of presence of metabolic alkalosis and/or HCO3− retention.
•The delta gap of less than 6 mEq/L is suggestive of presence of hyperchloremic acidosis and/or HCO3− excretion.
