
статьи / ᯨ᮪
.docx-
Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics
Volume 1647, Issues 1–2, 11 April 2003, Pages 193–199
Physiological and pathological implications of semicarbazide-sensitive amine oxidase
Peter H Yu, , Shannon Wright, Ellen H Fan, Zhao-Rong Lun, Diana Gubisne-Harberle
Abstract
Semicarbazide-sensitive amine oxidase (SSAO) catalyzes the deamination of primary amines. Such deamination has been shown capable of regulating glucose transport in adipose cells. It has been independently discovered that the primary structure of vascular adhesion protein-1 (VAP-1) is identical to SSAO. VAP-1 regulates leukocyte migration and is related to inflammation. Increased serum SSAO activities have been found in patients with diabetic mellitus, vascular disorders and Alzheimer's disease. The SSAO-catalyzed deamination of endogenous substrates, that is, methylamine and aminoacetone, led to production of toxic formaldehyde and methylglyoxal, hydrogen peroxide and ammonia, respectively. These highly reactive aldehydes have been shown to initiate protein cross-linkage, exacerbate advanced glycation of proteins and cause endothelial injury. Hydrogen peroxide contributes to oxidative stress. 14C-methylamine is converted to 14C-formaldehyde, which then forms labeled long-lasting protein adduct in rodents. Chronic methylamine treatment increased the excretion of malondialdehyde and microalbuminuria, and enhanced the formation of fatty streaks in C57BL/6 mice fed with an atherogenic diet. Treatment with selective SSAO inhibitor reduces atherogenesis in KKAy diabetic mice fed with high-cholesterol diet. Aminoguanidine, which blocks advanced glycation and reduces nephropathy in animals, is in fact more potent at inhibiting SSAO than its effect on glycation. It suggests that SSAO is involved in vascular disorders under certain pathological conditions. Although SSAO has been known for several decades, its physiological and pathological implications are just beginning to be recognized.
-
Tetrahedron Letters
Volume 47, Issue 49, 4 December 2006, Pages 8651–8652
Urea nitrate and nitrourea: powerful and regioselective aromatic nitration agents
Joseph Almoga, , , Asne Kleina, Anat Sokola, Yoel Sassona, Dana Sonenfeldb, Tsippy Tamirib
Abstract
Urea nitrate (UN) and nitrourea (NU), easily prepared from urea and nitric acid, convert deactivated aromatic compounds to the corresponding nitrated derivatives with a high yield and a high regioselectivity under very mild conditions. The performance of the two reagents is quite similar indicating that NU is an intermediate in the UN nitration process.
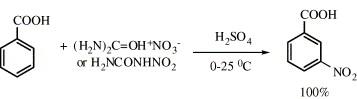
-
Russian Journal of General Chemistry
November 2006, Volume 76, Issue 11, pp 1719-1723
Synthesis and properties of Fe(II), Ni(II), Co(II), and Zn(II) chelates with 4-nitrosemicarbazide
S. G. Il’yasov, A. A. Lobanova, N. I. Popov, I. V. Kazantsev, V. L. Varand, S. V. Larionov
Abstract
Chelates M(O2NNCONHNH2)2 · 2H2O (M = Fe, Co, Ni, Zn) were prepared by the reactions of 4-nitrosemicarbazide with appropriate 3d transition metal salts. The structures of the compounds were suggested on the basis of data obtained by physicochemical methods. The compounds obtained were shown to be energetic substances.
-
Russian Journal of Organic Chemistry
December 2002, Volume 38, Issue 12, pp 1731-1738
Chemistry of Urea Nitro Derivatives: III. Reactions of N,N'-Dinitrourea with Bases
S. G. Il'yasov, A. A. Lobanova, N. I. Popov, R. R. Sataev
Abstract
N,N'-Dinitrourea reacts with bases to form the corresponding acid or neutral salts. Its reaction with hydrazine yields 4-nitrosemicarbazide, and the reaction with hydroxylamine leads to N-hydroxy-N'-nitrourea.
-
Propellants, Explosives, Pyrotechnics
Volume 38, Issue 3, pages 327–334, June 2013
Synthesis, Structure, and Properties of N,N′-Dinitrourea
Sergey G. Il'yasov1,*, Gennady V. Sakovich1 andAntonina A. Lobanova2
Abstract
Information on synthesis methods and properties of N,N′-dinitrourea and its salts, which were reported virtually simultaneously by different authors in different publications, is summarized and systematized. Merits and drawbacks of various approaches for the synthesis of the target products are discussed. The reactivity of N,N′-dinitrourea and its salts in the reactions of nucleophilic substitution and condensation is discussed.
-
Letters in Organic Chemistry, Volume 7, Number 3, April 2010, pp. 249-254(6)
Metal-Free Oxidation of Urazole and 1,4-Dihydropyridine Derivatives Under Mild and Heterogeneous Conditions by Nitro Urea, Derived from Urea Nitrate, and Silica Sulfuric Acid
Authors: Ghorbani-Choghamarani, Arash; A. Zolfigol, Mohammad; Hajjami, Maryam; Rastgoo, Shahrbanoo; Mallakpour, Shadpour
Abstract:
Mild combination of nitro urea, derived from urea nitrate, and silica sulfuric acid (SiO2OSO3H) might act as an efficient oxidizing media, which could be able to oxidize different types of heterocyclic compounds including urazoles and 1,4-dihydropyridines. The process presented here is operationally simple, environmentally benign, and reactions have been mildly carried out in dichloromethane at room temperature.
-
Tetrahedron
Volume 60, Issue 44, 25 October 2004, Pages 9953–9961
Reaction of 3-aminoquinoline-2,4-diones with nitrourea. Synthetic route to novel 3-ureidoquinoline-2,4-diones and imidazo[4,5-c]quinoline-2,4-diones
Antonín Kláseka, , , Antonín Lyčkab, Michal Holčapekc, Ignác Hozaa
Abstract
1-Unsubstituted 3-alkyl/aryl-3-amino-1H,3H-quinoline-2,4-diones react with 1-substituted and 1,1-disubstituted ureas in boiling acetic acid to give 2,6-dihydro-imidazo[1,5-c]quinazoline-3,5-diones. In contrast, the reaction of these amines with nitrourea in dioxane affords novel 3-alkyl/aryl-3-ureido-1H,3H-quinoline-2,4-diones or 9b-hydroxy-3a-alkyl/aryl-3,3a,5,9b-tetrahydro-1H-imidazo[4,5-c]quinoline-2,4-diones, which can smoothly be dehydrated to 3a-alkyl/aryl-3,3a-dihydro-5H-imidazo[4,5-c]quinoline-2,4-diones. All three types of products can be converted to 2,6-dihydro-imidazo[1,5-c]quinazoline-3,5-diones by refluxing in acetic acid.
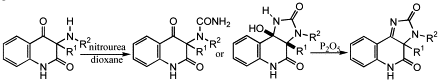
-
Journal of heterocyclic chemistry
2006, vol. 43, no5, pp. 1251-1260 [10 page(s) (article)]
Molecular rearrangement of 1-substituted 3-aminoquinoline-2,4-diones in their reaction with urea and nitrourea synthesis and transformations of reaction intermediates
KLASEK Antonin ; LYCKA Antonin ; HOLCAPEK Michal ; KOVAR Michal ; HOZA Ignac
-
Chemical Journal of Chinese Universities》 2010-12Add to Favorite Get Latest Update
3D-QSAR Study of N-Nitro Urea Derivatives with Herbicidal Activity
SHEN Xiao-Xia,XU Sheng-Zhen,CHEN Chang-Shui(Department of Chemistry,College of Science,Huazhong Agricultural University,Wuhan 430070,China)
Urea derivatives have occupied a pivotal position in pesticide chemistry due to their significant activities,including herbicidal activities,antimicrobial and so on.And N-nitro substituted anilines also displayed herbicidal properties.So we proposed that urea derivatives bearing a new group nitro in the NH—CO—NH bridge should display some interesting fungicidal activities.The preliminary bioassays indicated that N-nitro urea derivatives possess good activity against A.retroflexus L and S.sudanenses.To design the highly active title compounds,comparative molecular field analysis(CoMFA) was applied to the study of the three-dimensional quantitative structure activity relationship(3D-QSAR) on 38 N-nitro urea derivatives.The reasonable models with predictive ability were obtained from the investigation(A.retroflexus L:q2=0.674,r2=1.000,R2pred=0.9989;S.sudanenses:q2=0.635,r2=1.000,R2pred=0.9958).The steric and electronic contour maps show that bulky and electropositive groups linked to 2-,5-position of N'-phenyl ring,electronegetive groups to 3-position,small and electronegetive groups to 4-,6-position may increase the herbicidal activity against A.retroflexus L,and also bulky and electronegetive groups to 2-position,small and electronegetive groups to 3-position,bulky and electropositive groups to 4-position,bulky groups to 5-position may increase the activity against S.sudanenses.
-
Chinese Journal of Chemistry
Volume 29, Issue 4, pages 731–734, April, 2011
A Mild Procedure for the Preparation of o-Nitrophenols by Nitro Urea or Ammonium Nitrate in the Presence of Silica Sulfuric Acid (SiO2-OSO3H)
Arash Ghorbani-Choghamarani*, Mohsen Nikoorazm, Hamid Goudarziafshar, Zahra Naserifar andParisa Zamani
Abstract
A mixture of ammonium nitrate or nitro urea and silica sulfuric acid was found to be efficient and environmentally friendly nitrating media for the preparation of orto-nitro phenols in dichloromethane at room temperature.
-
Journal of Energetic Materials
Volume 26, Issue 3, 2008
A Theoretical Study on Nitrourea and its Tautomers
Abstract
In the present study, nitrourea and its possible tautomers have been subjected to theoretical analysis by performing Hartree-Fock and also density functional theory (DFT) calculations. The optimized geometries, vibrational frequencies, and some thermodynamical values for the presently considered species have been obtained in their ground states.
