
- •Contents
- •Contributors
- •Preface
- •1 Introduction, with the biological basis for cell mechanics
- •Introduction
- •The role of cell mechanics in biological function
- •Maintenance of cell shape
- •Cell migration
- •Mechanosensing
- •Stress responses and the role of mechanical forces in disease
- •Active cell contraction
- •Structural anatomy of a cell
- •The extracellular matrix and its attachment to cells
- •Transmission of force to the cytoskeleton and the role of the lipid bilayer
- •Intracellular structures
- •Overview
- •References
- •2 Experimental measurements of intracellular mechanics
- •Introduction
- •Forces to which cells are exposed in a biological context
- •Methods to measure intracellular rheology by macrorheology, diffusion, and sedimentation
- •Whole cell aggregates
- •Sedimentation of particles
- •Diffusion
- •Mechanical indentation of the cell surface
- •Glass microneedles
- •Cell poker
- •Atomic force microscopy
- •Mechanical tension applied to the cell membrane
- •Shearing and compression between microplates
- •Optical traps
- •Magnetic methods
- •Twisting of magnetized particles on the cell surface and interior
- •Passive microrheology
- •Optically detected individual probes
- •One-particle method
- •Two-particle methods
- •Dynamic light scattering and diffusing wave spectroscopy
- •Fluorescence correlation spectroscopy
- •Optical stretcher
- •Acoustic microscopy
- •Outstanding issues and future directions
- •References
- •3 The cytoskeleton as a soft glassy material
- •Introduction
- •Magnetic Twisting Cytometry (MTC)
- •Measurements of cell mechanics
- •The structural damping equation
- •Reduction of variables
- •Universality
- •Scaling the data
- •Collapse onto master curves
- •Theory of soft glassy rheology
- •What are soft glassy materials
- •Sollich’s theory of SGMs
- •Soft glassy rheology and structural damping
- •Open questions
- •Biological insights from SGR theory
- •Malleability of airway smooth muscle
- •Conclusion
- •References
- •4 Continuum elastic or viscoelastic models for the cell
- •Introduction
- •Purpose of continuum models
- •Principles of continuum models
- •Boundary conditions
- •Mechanical and material characteristics
- •Example of studied cell types
- •Blood cells: leukocytes and erythrocytes
- •Limitations of continuum model
- •Conclusion
- •References
- •5 Multiphasic models of cell mechanics
- •Introduction
- •Biphasic poroviscoelastic models of cell mechanics
- •Analysis of cell mechanical tests
- •Micropipette aspiration
- •Cells
- •Biphasic properties of the pericellular matrix
- •Indentation studies of cell multiphasic properties
- •Analysis of cell–matrix interactions using multiphasic models
- •Summary
- •References
- •6 Models of cytoskeletal mechanics based on tensegrity
- •Introduction
- •The cellular tensegrity model
- •The cellular tensegrity model
- •Do living cells behave as predicted by the tensegrity model?
- •Circumstantial evidence
- •Prestress-induced stiffening
- •Action at a distance
- •Do microtubules carry compression?
- •Summary
- •Examples of mathematical models of the cytoskeleton based on tensegrity
- •The cortical membrane model
- •Tensed cable nets
- •Cable-and-strut model
- •Summary
- •Tensegrity and cellular dynamics
- •Conclusion
- •Acknowledgement
- •References
- •7 Cells, gels, and mechanics
- •Introduction
- •Problems with the aqueous-solution-based paradigm
- •Cells as gels
- •Cell dynamics
- •Gels and motion
- •Secretion
- •Muscle contraction
- •Conclusion
- •Acknowledgement
- •References
- •8 Polymer-based models of cytoskeletal networks
- •Introduction
- •The worm-like chain model
- •Force-extension of single chains
- •Dynamics of single chains
- •Network elasticity
- •Nonlinear response
- •Discussion
- •References
- •9 Cell dynamics and the actin cytoskeleton
- •Introduction: The role of actin in the cell
- •Interaction of the cell cytoskeleton with the outside environment
- •The role of cytoskeletal structure
- •Actin mechanics
- •Actin dynamics
- •The emergence of actin dynamics
- •The intrinsic dynamics of actin
- •Regulation of dynamics by actin-binding proteins
- •Capping protein: ‘decommissioning’ the old
- •Gelsolin: rapid remodeling in one or two steps
- •β4-thymosin: accounting (sometimes) for the other half
- •Dynamic actin in crawling cells
- •Actin in the leading edge
- •Monomer recycling: the other ‘actin dynamics’
- •The biophysics of actin-based pushing
- •Conclusion
- •Acknowledgements
- •References
- •10 Active cellular protrusion: continuum theories and models
- •Cellular protrusion: the standard cartoon
- •The RIF formalism
- •Mass conservation
- •Momentum conservation
- •Boundary conditions
- •Cytoskeletal theories of cellular protrusion
- •Network–membrane interactions
- •Network dynamics near the membrane
- •Special cases of network–membrane interaction: polymerization force, brownian and motor ratchets
- •Network–network interactions
- •Network dynamics with swelling
- •Other theories of protrusion
- •Numerical implementation of the RIF formalism
- •An example of cellular protrusion
- •Protrusion driven by membrane–cytoskeleton repulsion
- •Protrusion driven by cytoskeletal swelling
- •Discussion
- •Conclusions
- •References
- •11 Summary
- •References
- •Index
Cell dynamics and the actin cytoskeleton |
183 |
is comparable to the time between monomer assembly events in most experiments, Pollard’s assumption that ATP-actin was the disassembling species in his experiments must be reevaluated. Also significant were data revealing that inorganic phosphate was released from the cleaved nucleotide several minutes after hydrolysis (Carlier and Pantaloni, 1986). The latter discovery meant that three species needed to be considered for actin dynamics: ATP, ADP, and a long-lived intermediate ADP·Pi. Furthermore, an ADP·Pi monomer species should be generated by disassembly to either reassemble or release inorganic phosphate (Pi) and become a source of ADP-G-actin. Unfortunately, there has been no focused effort to determine all twelve assembly/disassembly rate constants and the two rates of Pi release (G-actin and F-actin) needed to properly update Oosawa’s model. One reason for the missing effort is that both biochemical and structural data indicate that ADP·Pi and ATP-F-actin are similar (Otterbein et al., 2001; Rickard and Sheterline, 1986; Wanger and Wegner, 1987), so that distinguishing between the two species may be unnecessary in many contexts. Assuming equivalence between ADP·Pi and ATP-actin species, we have recently published a broad mathematical model of the steady-state actin cycle that predicts a broad range of experimentally observed behaviors (Bindschadler et al., 2004). While this agreement is encouraging, it does not replace the need for newly designed experiments that definitively establish rates.
In Bindschadler et al. (2004) we carefully tabulated consensus rate constants for intrinsic actin dynamics. While controversies persist concerning the mechanism of ATP hydrolysis and the rate of nucleotide exchange on G-actin, a growing consensus on these topics can be inferred from agreements by independent laboratories. As mentioned, the rates for the ADP·Pi-actin species remain unmeasured. Also unaddressed is the fact that the rate constants must depend on the nucleotide content of filaments and so the single values reported by Pollard cannot constitute the complete story.
Regulation of dynamics by actin-binding proteins
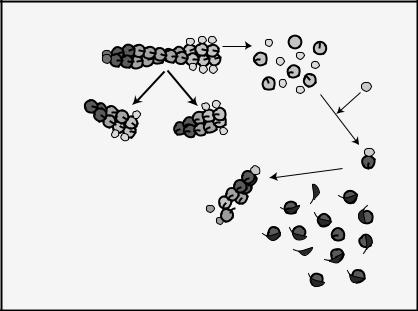
Many efforts over the past twenty years have focused on elucidating the mechanisms by which actin-associated proteins modulate actin dynamics. These efforts are essential because intracellular signaling pathways do not modify actin itself, and so the regulation of binding proteins provides an indirect route for changing cytoskeletal structure and cell shape. Unlike solutions of pure actin, cells contain short, dynamic filaments in highly structured networks and often a large fraction of unpolymerized actin. Here we review properties of actin-binding proteins thought to account for the major differences between the dynamics of cellular and purified actin (see Fig. 9-7).
ADF/cofilin: targeting the rate-limiting step in the actin cycle
Named for their activity as “actin depolymerizing factors” and their ability to form cofilamentous structures with F-actin, the ADFs and cofilins form two subgroups of a family of proteins (ADF/cofilins or ACs) expressed in most mammalian cells. Efforts to understand AC function have been challenged by a multiplicity of AC functions that differ slightly between the ADFs and cofilins and also among species (Bamburg, 1999). In general, ACs bind to both monomeric and filamentous actin with rather
184 J.L. McGrath and C.F. Dewey, Jr.
enhanced ADP-actin barbed end capping disassembly by cofilin by capping protein 
and gelsolin
|
enhanced nucleotide |
|
exchange and |
filament severing by |
polymerization by profilin |
cofilin and gelsolin |
|
new filament generation by 

 Arp2/3 complex, gelsolin
Arp2/3 complex, gelsolin
monomer sequestration by β-thymosin
Fig. 9-7. Regulation of actin dynamics. Actin-binding proteins modulate every phase of the actin cycle including assembly and disassembly kinetics, nucleotide exchange, and filament number and length.
exclusive affinity for the ADP-bound conformations (Carlier et al., 1997; Maciver and Weeds, 1994). On filaments, structural data indicate that ACs bind the sides of filaments and destablize the most interior interactions between subunits (Bobkov et al., 2004; Galkin et al., 2003; McGough et al., 1997). AC-decorated filaments break along their length (Maciver et al., 1991) and rapidly disassemble at their ends (Carlier et al., 1997). Despite debate over whether ACs should be thought of primarily as filament-severing proteins or catalysts of ADP subunit disassociation (Blanchoin and Pollard, 1999; Carlier et al., 1997), both effects may occur as manifestations of the same structural instability on filaments. ACs have been shown to increase the rate of Pi release on filaments, and to slow the rate at which ADP monomers recharge with ATP on monomer (Blanchoin and Pollard, 1999). Thus ACs both hasten the production of ADP-actin and stabilize the ADP form. Some AC proteins bind ADP-G-actin with a much higher affinity than ADP-F-actin to create a thermodynamic drive toward the ADP-G-actin state (Blanchoin and Pollard, 1999). Like their multiple functions, ACs have multiple avenues for regulation including pH sensitivity, inactivation by PIP2 binding, and serine phosphorylation (Bamburg, 1999).
Given this seemingly perfect arsenal of disassembling functions in vitro, observations that ACs trigger polymerization and generate new barbed ends in cells (Ghosh et al., 2004) certainly appear contradictory. There are at least two likely explanations for the paradox. First, with a large pool of sequestered ATP-actin available to assemble at free barbed ends (see the discussion of thymosins), the conditions inside a cell are primed for assembly (Condeelis, 2001). Thus filaments generated by AC severing may not have sufficient time to disassemble before they become nuclei for new filament
Cell dynamics and the actin cytoskeleton |
185 |
growth. Supporting this, enhanced assembly occurs at early time points in vitro when ACs are added to solutions containing an excess of ATP-G-actin. (Blanchoin and Pollard, 1999; Du and Frieden, 1998; Ghosh et al., 2004).
The cellular data on cofilin-mediated growth should not be interpreted to mean that ACs are not involved in filament dissolution in vivo. Indeed, theoretical calculations indicate that severing alone cannot explain how filament turnover in cells occurs orders of magnitude faster than unregulated actin (Carlier et al., 1997). Thus, the second explanation for the paradox is that AC-mediated disassembly is directly linked to filament assembly. If pointed-end disassembly is rate-limiting for the actin cycle in vivo as it is in vitro, the enhanced production of ADP-G-actin should lead to a larger supply of ATP-monomer and enhanced polymerization elsewhere. Thus both of the ‘destructive’ activities of ACs – severing and enhanced disassembly – can lead to filament renewal and rapid turnover in the cellular environment.
Profilin: a multifunctional protein to close the loop
Profilin was the first monomer-binding protein discovered and originally thought to sequester G-actin in a nonpolymerizable form (Tobacman and Korn, 1982; Tseng and Pollard, 1982). However, later data made clear that profilins do not prevent actin assembly, but actually drive the assembly phase of the actin cycle (Pollard and Cooper, 1984). Today profilins are known to catalyze the rate of nucleotide exchange on G-actin by as much as an order of magnitude (Goldschmidt-Clermont et al., 1991; Selden et al., 1999). In cells this means that newly released ADP-G-actin is recharged to the ATP state shortly after binding to profilin. Significantly, the profilin-G-actin complex is capable of associating with filament barbed ends, but not with pointed ends (Pollard and Cooper, 1984). Profilin binds to a structural hinge on the barbed end of an actin filament and slightly opens the hinge to expose the nucleotide-binding pocket and promote nucleotide exchange (dos Remedios et al., 2003; Schutt et al., 1993). Profilin binding in this region also sterically blocks association of G-actin with pointed ends. Because there is no evidence that profilin blocks actin assembly at barbed ends, profilin presumably instantly disassociates from monomer after assembly. Further, the profilin-actin complex assembles at barbed ends at the same rates as ATP-G-actin alone (Kang et al., 1999; Pantaloni and Carlier, 1993). With these properties, profilin-bound actin becomes a subpopulation of barbed-end- specific monomer (Kang et al., 1999). In our published analysis of the actin cycle (Bindschadler et al., 2004), we found that profilin’s functions provide the perfect complement to cofilin’s disassembly functions. Together the two proteins appear to overcome every major barrier to increasing the rate of filament treadmilling (Bindschadler et al., 2004).
Arp2/3 complex and formins: making filaments anew
A perplexing and important question for cell biologists in the 1980s and 1990s was “how are new filaments created in cells?” One answer was that new filaments are generated when existing filaments are first severed and then elongate; however there was no reasonable mechanism for the de novo generation of filaments in cells. In the late 1990s it became clear that the Arp2/3 complex was dedicated to this task (Mullins et al., 1998; Pollard and Beltzner, 2002). The two largest members of this
186J.L. McGrath and C.F. Dewey, Jr.
seven-protein complex are the actin-related proteins Arp2 and Arp3 (Machesky et al., 1994). Like the other members of the Arp family, Arp2 and Arp3 share a strong structural similarity to actin. In the Arp2/3 complex, these similarities are used to create a pocket that recruits an actin monomer to form a pseudotrimer that nucleates a new filament (Robinson et al., 2001). The complex holds the growing filament at its pointed end, leaving the barbed end free for rapid assembly (Mullins et al., 1998). For robust nucleation of filaments, the Arp2/3 complex requires activation, first by WASp/Scar family proteins (Machesky et al., 1999), and secondarily by binding to preexisting F-actin (Machesky et al., 1999). The arrangement gives autocatalytic
growth of branched filament networks in vitro: new filaments grow from the sides of old ones to create a 70◦ included angle (Mullins et al., 1998). This same network
geometry is found at the leading edge of cells and the Arp2/3 complex localizes to branch points in the cellular networks (Svitkina and Borisy, 1999).
While it is now clear that the Arp2/3 complex is an essential ingredient of the actin cytoskeleton, its discovery is new enough that many details of its mechanism are clouded in controversy. The most visible controversy has been over the nature of the Arp2/3 complex/F-actin interaction. Carlier and colleagues argue that the complex incorporates at the barbed ends of actin filaments to create a bifurcation in filament growth (Pantaloni et al., 2000); however, using direct visualization of fluorescently labeled filaments, several laboratories have demonstrated that new filaments can grow from the sides of preexisting filaments (Amann and Pollard, 2001a; Amann and Pollard, 2001b; Fujiwara et al., 2002; Ichetovkin et al., 2002). One paper appears to resolve the confusion with data indicating that branching occurs primarily from the sides of ATP-bound regions of the mother filament very near the barbed end (Ichetovkin et al., 2002). However, others believe that branching can occur on any subunit but filaments release or debranch rapidly from ADP segments of the mother filaments. This idea is supported by a correlation between the kinetics of debranching and Pi release on the mother filament (Dayel and Mullins, 2004), but contradicted by a report indicating that ATP hydrolysis on Arp2 is the trigger for debranching (Le Clainche et al., 2003). A disheartened reader looking for clearer understandings should consult the most recent reviews on the Arp2/3 complex.
Very recently, it has become clear that the Arp2/3 complex is not the only molecule capable of de novo filament generation in cells. In yeast, members of the formin family of proteins generate actin filament bundles needed for polarized growth (Evangelista et al., 2002), and in mammalian cells formin family members help generate stress fibers (Watanabe et al., 1999) and actin bundles involved in cytokinesis (Wasserman, 1998). Dimerized FH2 domains of formins directly nucleate filaments in a most remarkable manner (Pruyne et al., 2002; Zigmond et al., 2003). The FH2 dimer remains attached to the barbed ends of actin filaments even as it allows insertion of new subunits at that same end (Kovar and Pollard, 2004; Pruyne et al., 2002). By tracking the barbed ends of growing filaments, formins block associations with capping protein (Kovar et al., 2003; Zigmond et al., 2003). In cells where capping protein and cross-linking proteins are abundant, forminbased nucleation should naturally lead to bundles of long filaments, while Arp2/3- complex-generated filaments should naturally arrange into branched networks of short filaments.
