Intermediate Physics for Medicine and Biology - Russell K. Hobbie & Bradley J. Roth
.pdf






500 |
17. Nuclear Physics and Nuclear Medicine |
|
|
|
|
||
|
|
TABLE 17.5. Some typical doses for nuclear medicine procedures. |
|
||||
|
|
|
|
|
|
|
|
|
|
Study and agent |
A0 (MBq) |
Organ and high- |
Total body |
E ective |
|
|
|
|
|
est dose (mSv) |
|
dose (mSv) |
dose (mSv) |
|
|
|
|
|
|
|
|
|
|
Bone |
555 |
Bladder wall |
51 |
2.0 |
4.4 |
|
|
99mTc–pyrophosphate |
|
|
|
|
|
|
|
Heart |
55 |
Kidneys |
20 |
3.6 |
13 |
|
|
201Tl–chloride |
|
|
|
|
|
|
|
Liver |
185 |
Bladder wall |
17 |
0.9 |
2.6 |
99mTc–sulfur colloid
Source: Adapted from Table 9-3 in P. B. Zanzonico, A. B. Brill, and D. V.
Becker, Radiation dosimetry, Chapter 9 in H. N. Wagner, Jr., Z. Szabo, and
J. W. Buchanan, eds. Principles of Nuclear Medicine, 2nd. ed. Philadelphia,
Saunders (1995).
s are shown on the bottom lines. The dose to the lungs is 4.3 ×10−3 Gy. The whole body dose is much less because the absorbed energy is divided by the mass of the entire body. The value of φ shows that about 38% of the photon energy is absorbed in the body.
The dose to the lungs is considerably greater than in a |
|
|
|
chest x ray; however, a chest x ray is almost useless for |
|
|
|
diagnosing a pulmonary embolus. The whole body dose |
|
|
|
is not unreasonable. |
|
|
|
Table 17.5 shows some typical doses from various nu- |
|
|
|
clear medicine procedures. The e ective dose is defined |
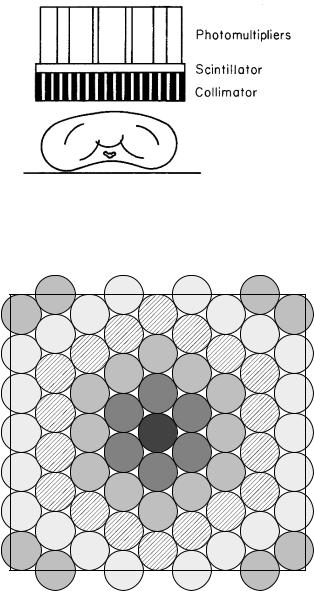
FIGURE 17.16. A scintillator with a lead collimator to give |
||
on p. 468. |
|||
directional sensitivity. |
|||
|
|||
17.11 Auger Electrons |
is about 4. The fraction of the Auger emitter that binds |
||
to the DNA depends on the chemical agent to which the |
|||
In Sec. 15.9 we discussed the deexcitation of atoms, in- |
nuclide is attached. There is also a significant bystander |
||
e ect [Kassis (2004)]. |
|||
cluding the emission of Auger and Coster–Kronig elec- |
|
|
|
trons. The Auger cascade means that several of these |
17.12 Detectors; The Gamma Camera |
||
electrons are emitted per transition. If a radionuclide is |
|||
in a compound that is bound to DNA, the e ect of sev- |
Nuclear medicine images do not have the inherent spa- |
||
eral electrons released in the same place is to cause as |
|||
much damage per unit dose as high-LET radiation. Lin- |
tial resolution of diagnostic x-ray images; however, they |
||
ear energy transfer was defined in Chapter 15. A series of |
provide functional information: the increase and decrease |
||
reports on this e ect have been released by the American |
of activity as the radiopharmaceutical passes through the |
||
Association of Physicists in Medicine (AAPM) [Sastry |
organ being imaged. |
||
(1992); Howell (1992); Humm et al. (1994)]. |
Early measurements were done with single detectors |
||
Many electrons (up to 25) can be emitted for one |
such as the scintillation detector10 shown in Fig. 17.16. |
||
nuclear transformation, depending on the decay scheme |
Directional sensitivity is provided by a collimator, which |
||
[Howell (1992)]. The electron energies vary from a few eV |
can be cylindrical or tapered. Single detectors are still |
||
to a few tens of keV. Corresponding electron ranges are |
used for in vitro measurements and for thyroid uptake |
||
from less than 1 nm to 15 µm. The diameter of the DNA |
studies. |
||
double helix is about 2 nm. A number of experiments [re- |
Two-dimensional images can be taken with the scin- |
||
viewed in the AAPM reports, also Kassis (2004)] show |
tillation camera or gamma camera shown in Figs. 17.17 |
||
that when the radioactive substance is in the cytoplasm |
and 17.18. The scintillator is 6–12 mm thick and about 60 |
||
the cell damage is like that for low-LET radiation in Fig. |
cm across. Modern scintillators are rectangular. The scin- |
||
15.32 with relative biological e ectiveness (RBE) = 1. |
tillator is viewed by an array of 50–100 photomultiplier |
||
When it is bound to the DNA, survival curves are much |
tubes arranged in a hexagonal array. The tube nearest |
||
steeper, as with the α particles in Fig. 15.32 (RBE ≈ 8). |
|
|
|
When it is in the nucleus but not bound to DNA the RBE |
10Scintillation detectors were discussed in Sec. 16.3. |
||