
- •Lab 2. Copper plating of the copper plate and proof of the Faraday's Law Mashtakova k.H., Ahmadiyarova z.K., Sertaeva z.O.
- •We derive Faraday's law, based on our knowledge
- •3. Advantages and disadvantages of copper plating electrolytes.
- •4. Options:
- •5. Reagents and Equipment:
- •6. Progress of the work:
- •Figure 1. Before the electrolysis
- •Figure 2. Electrolysis of copper products
- •Figure 3. After the electrolysis
4. Options:
– room temperature;
– time (5 min);
– current was varied (0.5 а; 1.0 а; 1.5 a);
– an electrolyte (solution).
5. Reagents and Equipment:
СuSO₄*5Н2О – 150 g/l;
H₂SO₄- 50 g/l;
copper electrode;
copper plate (S = 9 cm2);
500 ml flask;
distilled water;
mount (end sleeves);
copper wire;
power supply;
electrolytic cell;
lacquer;
a tripod.
6. Progress of the work:
1. Prepare the electrolyte, i.e., solution. Take 75 g CuSO₄ and diluted in a glass of 500 ml of distillate. Add water and 25 g/ml H₂SO₄. Mix solution until complete dissolution of the precipitate.
2. Putting the chain. At the cathode: copper products.
At the anode: Сu.
3. Treat the product with sandpaper, isolate one side of the nail, and dry them. Washed and treated with the product in 0.01 N HCl, washed in distilled water. The copper electrode is used as the cathode, dry them and weigh on the laboratory scale, record the measured weight of the M1. We perform electrolysis at different currents within 5 minutes. After completion of the electrolysis process, the product is washed with water and dried. Then the obtained product is weighed on an analytical balance. Write the value of M2. The difference M2 - M1 increments cathode mass M.

Figure 1. Before the electrolysis
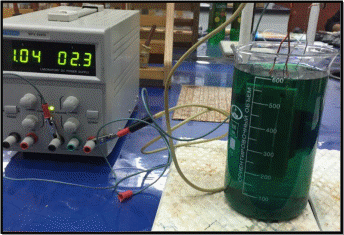
Figure 2. Electrolysis of copper products



1 – 0,5 А 2 – 1,0 А 3 – 1,5 А
Figure 3. After the electrolysis
7. Calculation:
Calculation of mass (practical) at a current of 0.5 A:
m (w) = 1.9904 g
m (after) = 2.0403 g
m (Cu) = 2,0403 – 1,904 = 0,049 g
2) Calculation of mass (practical) at a current of 1.0 A:
m (w) = 2.0478 g
m (after) = 2.1413 g
m (Cu) = 0,094 g
3) Calculation of mass (practical) at a current of 1.5 A:
m (w) = 2.4008 g
m (after) = 2.5208 g
m (Cu) = 0,12 g
4) Calculation of mass (theoretical):
m (1) = q · I · τ = 1,19 g/Ah · 0.5 A · 0.083 h = 0.049 g
m (2) = 1.19 g/A·h · 1.0 A · 0.083 h = 0,099 g
m (3) = 1.19 g/A·h · 1.5 A · 0.083 h = 0.14 g

8. Conclusion
The English physicist Michael Faraday established electrolysis law experimentally in 1833. Faraday's Law defines the number of primary products, released at the electrodes during electrolysis. Products electrolyte decomposition in the experience stand out on the electrodes as long as there is current. The mass of released substance can be measured. If we choose such a solution in which the substance is liberated in the form of a solid deposited precipitate on the electrode, then the weight can be measured easily. Thus, if the current flowing through a solution of copper sulfate (CuSO4), the copper deposited on the cathode. If the cathode is weighed before and after the experiment, it is possible to accurately determine the mass of the deposited metal.
Measurements show that the mass of substance liberated at the electrodes depends on the current intensity and the time of electrolysis. At constant time, you can make sure that the mass of the extracted material is proportional to the amount of current flowing. In order to establish, as it depends on the current strength, we conducted experiments. We have made several identical in form and nature of the three products. Since the process is steady, at the same time asked the various amperage.
If you miss a period of time t in terms of copper sulfate solution a small current, the cathode is covered with little copper, and if the current is greater force, for, while at the cathode stand out more and more copper. Notice that copper stands out even more. Passing through different electrolytes different currents and carefully measuring the mass of a substance released at the electrodes of each of the electrolyte, Faraday discovered the first law of electrolysis. Each ion carries a certain mass and charge quantity of substance and, therefore, more ions than the solution to the electrode that is greater than the current in the electrolyte; the more the electrode material is released. Thus, experience shows that the mass of substance liberated at the electrodes during electrolysis is directly proportional to the product of the current strength of its passage through the electrolyte.
